Table of Contents
An Overview of α-Synuclein oligomers and Its Involvement in Neurodegenerative Disorders:
Scientists have extensively studied the complex and diverse nature of neurodegenerative disorders for a long time. Out of the many proteins involved in these circumstances, α-Synuclein oligomers is especially noteworthy. This compact, easily dissolvable protein has attracted significant interest because of its connection with Parkinson’s disease (PD), a severe condition that impacts millions of people across the globe. In addition to Parkinson’s disease, α-Synuclein is also involved in a range of other neurodegenerative disorders, commonly referred to as synucleinopathies. These disorders include Lewy body dementia and multiple system atrophy.
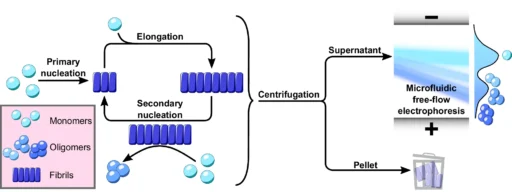
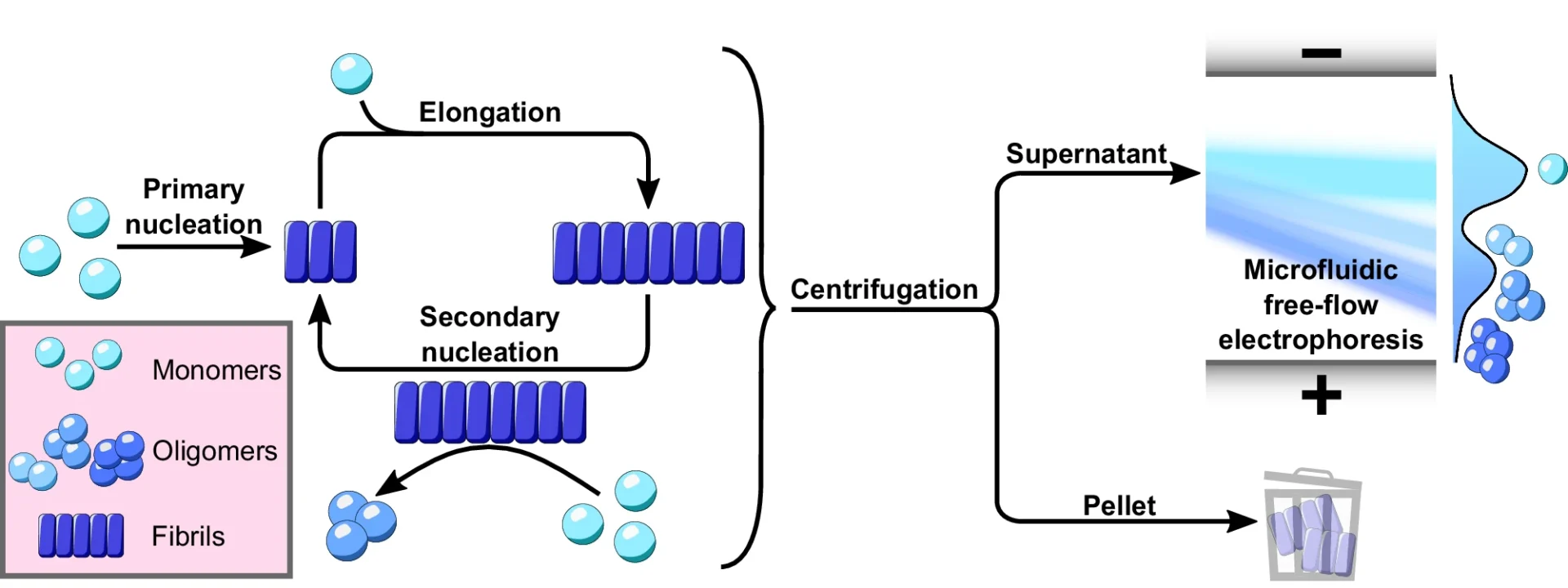
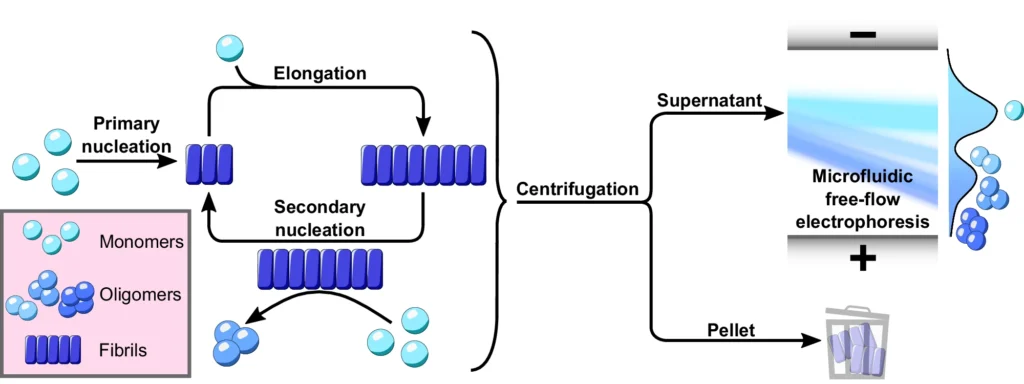
The defining characteristic of these illnesses is the atypical buildup of α-Synuclein oligomers aggregates in the brain, resulting in the gradual deterioration of neurons. Nevertheless, comprehending the exact mechanisms that propel α-Synuclein to create these detrimental aggregates continues to be a crucial obstacle in the field. A new study found that secondary nucleation is a key step in the formation of poisonous α-Synuclein oligomers, which are thought to play a major role in the development of many disorders. Using microfluidic free-flow electrophoresis with single molecule detection, we were able to fractionate α-synuclein aggregation mixtures and determine oligomer dynamics.
Understanding the protein aggregation process:
To completely comprehend the importance of α-Synuclein oligomers aggregation, it is crucial to first have a comprehensive understanding of the more general occurrence of protein aggregation. Proteins are intricate compounds made up of amino acids, which arrange themselves into precise three-dimensional configurations to carry out their tasks. Proteins tightly control their folding process, and their precise conformation is essential for their proper functioning. Nevertheless, proteins have the potential to undergo misfolding in specific circumstances, resulting in the creation of aggregates, which are aberrant groups of proteins that stick together.
Protein aggregation is a phenomenon that happens in various neurodegenerative disorders, such as Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). Under each of these circumstances, distinct proteins form aggregates in the brain, disrupting cellular function and ultimately resulting in cell death. The main part of the aggregates in synucleinopathies like Parkinson’s disease is the protein α-Synuclein. These aggregates are made up of structures inside neurons called Lewy bodies and Lewy neurites.
The structure and function ofα-Synuclein oligomers:
Synuclein, a protein with 140 amino acids, is primarily found in the brain. It plays a crucial role in synaptic activity. Although α-Synuclein has been extensively researched, its precise physiological function remains incompletely understood. We recognize it for its role in controlling the release of neurotransmitters, specifically dopamine, and believe it plays a role in synaptic plasticity, the capacity of synapses to enhance or diminish over time, which is crucial for learning and memory.
The classification of synuclein as an intrinsically disordered protein (IDP) indicates that it lacks a specific or stable three-dimensional structure in normal physiological settings. Instead, it exists as a complex and ever-changing collection of different shapes and structures. The normal function of α-Synuclein oligomers is thought to rely on its structural flexibility, which enables it to interact with various proteins and membranes. Nevertheless, this inherent flexibility also renders α-Synuclein susceptible to misfolding and aggregation.
Pathologically, α-Synuclein oligomers can form different structures, such as tiny oligomers, bigger fibrils, and ultimately, Lewy bodies. Each of these types has distinct implications for the disease’s progression. Fibrils and Lewy bodies are thought to be the more advanced and visible forms of α-Synuclein aggregation. However, new research suggests that smaller, soluble oligomers may be the more harmful entities, playing a key role in the early stages of neurodegenerative disorders. Secondary processes are involved in the aggregation of α-synuclein.
Pathways of α-Synuclein Aggregation:
The process of α-Synuclein aggregation is intricate and involves multiple steps. It begins with the formation of tiny, soluble aggregates known as nucleation seeds. The first stage, referred to as primary nucleation, entails the spontaneous formation of α-Synuclein monomers into tiny aggregates. After the formation of these seeds, they can increase in size by enlisting more α-Synuclein monomers. This leads to the extension of fibrils.
Nevertheless, new research has uncovered that the major nucleation pathway is not the sole mechanism by which α-Synuclein oligomers aggregate. Secondary nucleation, sometimes referred to as a secondary route, is a crucial factor in the creation of α-Synuclein oligomers. Secondary nucleation is distinct from primary nucleation since it does not entail the spontaneous production of aggregates in a solution. However, it takes place only on the outer layer of preexisting fibrils, where these fibrils act as catalysts for the creation of fresh oligomers.
Concerns arise from secondary nucleation’s potential to rapidly increase the number of harmful oligomers, believed to be the primary drivers of neurodegeneration in synucleinopathies. This procedure not only speeds up the accumulation of α-Synuclein but also aids in the dissemination of disease inside the brain, as these recently created clusters might disseminate the accumulation process to other areas.
Secondary nucleation is an essential process in the formation of α-synuclein oligomers:
Secondary nucleation plays a crucial role in the process of α-Synuclein oligomers aggregation and is different from the primary nucleation route. During secondary nucleation, α-Synuclein fibrils that are already there are used as a guide or catalyst to help make new oligomers from single α-Synuclein molecules. The process takes place on the surface of fibrils, where individual molecules attach to specific locations, undergo structural modifications, and create groups of molecules called oligomers.
The formation of oligomers can greatly accelerate due to the extremely effective surface-catalyzed reaction. Secondary nucleation is widely believed to play a significant role in the rapid advancement of synucleinopathies. The oligomers that are made through secondary nucleation are more harmful because they are more likely to interact with cell membranes, stop cells from working, and cause them to die.
The identification of secondary nucleation as a critical mechanism in the aggregation of α-Synuclein has significant implications for our understanding of the illness progression. It shows that the buildup of α-Synuclein oligomers in the brain is not just caused by proteins misfolding, but is a dynamic process that is affected by the catalytic activity of fibrils that were already there. Understanding this discovery has opened up new possibilities for medical treatment, as focusing on secondary nucleation can stop or delay the progression of neurodegenerative illnesses.
Exploring the Molecular Mechanisms of Secondary Nucleation:
At the molecular level, secondary nucleation is caused by the complex interaction between α-Synuclein monomers and fibrils that were already there. Upon encountering the fibril surface, monomers undergo a conformational shift that enhances their propensity to aggregate. The process is not simply a passive binding of monomers to fibrils; it entails a catalytic reaction in which the fibril surface speeds up the creation of new oligomers.
Surface-templated reactions are a crucial aspect of secondary nucleation. It is easier for individual α-Synuclein oligomers molecules to change into shapes that make them more likely to stick together when they hit the surface of the fibril. It is believed that this templating effect occurs through specific contacts between the monomers and the fibril’s surface. These interactions help stabilize intermediate forms that are susceptible to additional aggregation.
As a result, the newly formed oligomers can separate from the fibril and continue the aggregation process in other regions of the brain. The ability to produce fresh seeds for aggregation is an important factor in the spread of α-Synuclein oligomers disease. Additionally, the oligomers formed through secondary nucleation are structurally different from those formed through primary nucleation, which could make them more toxic.
Recent research has indicated that particular areas inside the α-Synuclein molecule might have an impact on secondary nucleation. Specific segments of α-Synuclein are more susceptible to structural alterations that encourage the formation of aggregates. These segments likely have a significant impact on the process of secondary nucleation. Gaining knowledge about the specific factors that influence secondary nucleation could offer significant information for developing therapeutic approaches that focus on this mechanism. α-Synuclein oligomers form by secondary nucleation.
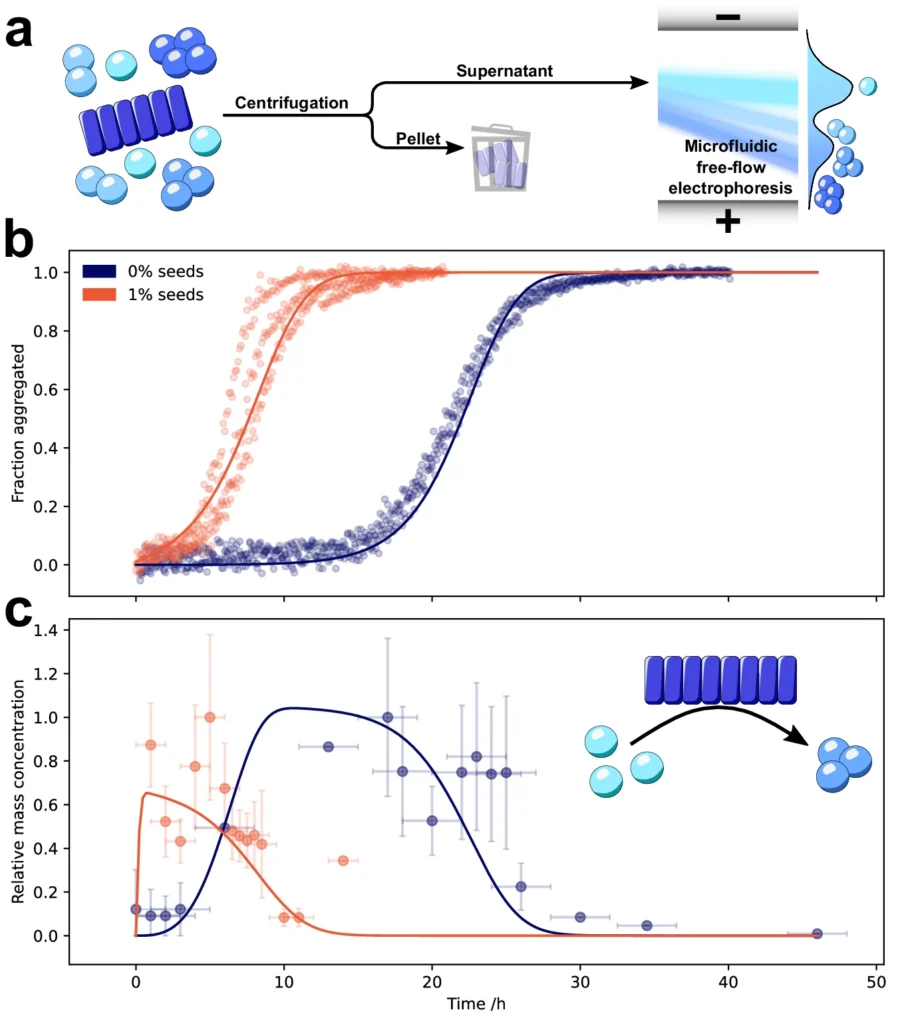
Significance of Oligomerization in Disease Pathogenesis:
The process of α-Synuclein oligomerization by secondary nucleation has important consequences for the development of neurodegenerative disorders. While fibrils and Lewy bodies have long been considered the primary pathogenic features of synucleinopathies, recent research suggests that oligomers could pose the greatest threat during the aggregation process.
Significance of Oligomerization in Disease Pathogenesis:
Oligomers exhibit a high level of reactivity and can interact with cellular membranes, disrupting cellular homeostasis. One of the most well-known harmful consequences of α-Synuclein oligomers is their capacity to create pores in cellular membranes. These pores facilitate the unregulated entry of calcium ions into the cell, which can initiate a series of reactions leading to cell death. This mechanism is especially significant in Parkinson’s disease, as the degeneration of dopaminergic neurons in the substantia nigra is a characteristic feature of the condition.
α-Synuclein oligomers not only have direct harmful effects, but they can also facilitate the aggregation process by initiating the development of additional aggregates in nearby cells. The prion-like behavior is believed to play a role in the dissemination of α-Synuclein oligomers disease across the brain, which is responsible for the gradual progression of synucleinopathies.
Studies have demonstrated a correlation between the amounts of oligomers and the severity of neurodegenerative disorders, providing additional evidence for the role of oligomers in these conditions. For instance, the brains of patients with severe Parkinson’s disease have shown elevated quantities of α-Synuclein oligomers in comparison to individuals in the early stages of the condition. This correlation implies that oligomers could be a catalyst for disease progression.
Because of their significant role in disease development, there has been a strong emphasis on studying α-Synuclein oligomers for therapeutic purposes. Researchers are currently aggressively studying strategies to decrease the development of oligomers, improve their removal, or counteract their harmful consequences as potential therapies for synucleinopathies.
Variables Affecting Secondary Nucleation:
Several factors influence the effectiveness and rate of secondary nucleation in α-Synuclein aggregation. Gaining a comprehensive understanding of these elements is essential for formulating effective techniques to regulate or suppress this process.
α-Synuclein Concentration: The level of α-Synuclein concentration plays a crucial role in the process of secondary nucleation. Higher levels of α-Synuclein make it more likely for monomers to interact with fibrils, which speeds up the formation of oligomers. The connection between concentration and protein aggregation is a common feature of the process. This shows how important it is to keep α-Synuclein homeostasis for the health of neurons.
Environmental Factors: Environmental factors, such as pH, temperature, and the presence of metal ions, can also influence secondary nucleation. For example, research has shown that certain metal ions, such as copper and iron, may speed up the process of α-Synuclein clumping together by keeping stable intermediate forms that are more likely to start nucleation. Fluctuations in pH can impact the distribution of charges on the α-Synuclein molecule, hence modifying its tendency to form aggregates. These environmental factors could potentially influence the differences in α-Synuclein oligomers aggregation seen in neurodegenerative disorders, both in terms of location and time.
Post-translational changes (PTMs): α-Synuclein oligomers experience multiple post-translational changes, such as phosphorylation, nitration, and truncation. These alterations can have a significant impact on α-Synuclein’s aggregation characteristics and secondary nucleation. It has been shown that phosphorylation at Serine 129, which is a change that happens a lot in Parkinson’s disease, makes α-Synuclein stick together more. Likewise, removing the C-terminal portion of α-Synuclein enhances its tendency to aggregate. Gaining knowledge about the influence of PTMs on secondary nucleation could provide a valuable understanding of the molecular processes involved in illness progression and help identify potential targets for therapeutic interventions.
Fibril Structure: The arrangement of preexisting fibrils is also critical in the secondary nucleation process. Fibers can take on various polymorphic forms, each of which has unique structural and metabolic characteristics. These different polymorphs can vary in their capacity to catalyze secondary nucleation, with certain forms exhibiting greater efficacy in stimulating the formation of oligomers compared to others. The presence of different fibril structures may contribute to the differences observed in synucleinopathies and the varying ways in which the disease manifests in different people. Secondary nucleation proceeds under quiescent, physiological conditions.
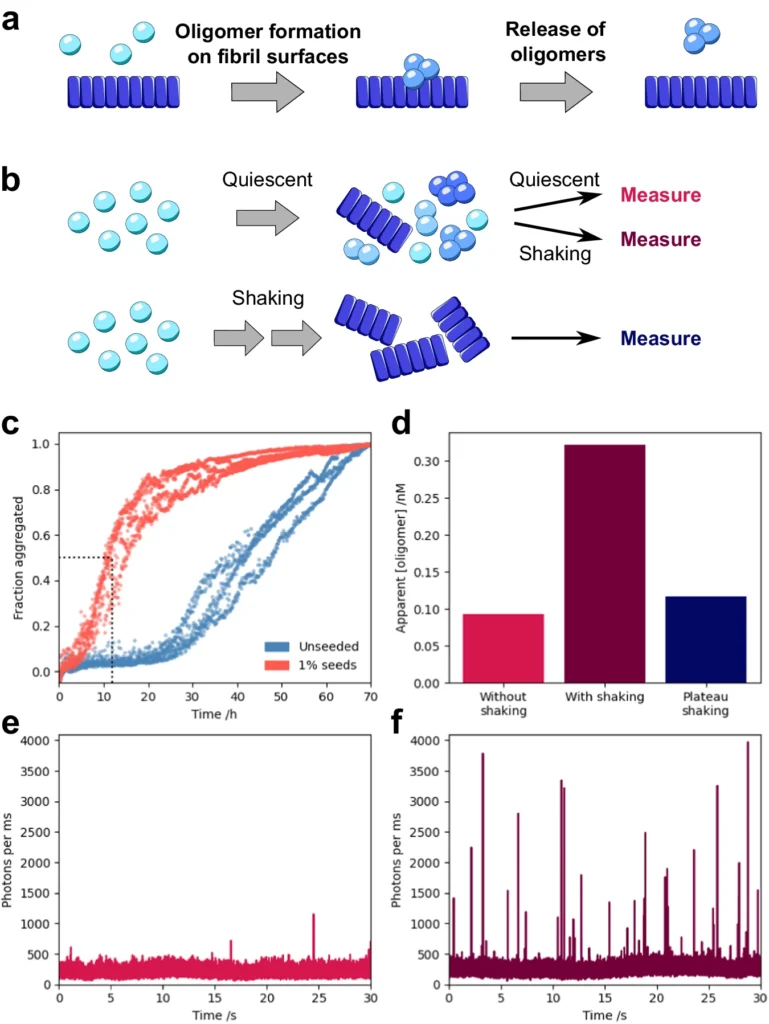
Methods for Investigating α-Synuclein oligomers:
It’s hard to study α-Synuclein oligomerization, also known as secondary nucleation, because the oligomers are short-lived and have different properties. Nevertheless, advancements in biophysical and molecular methodologies have empowered researchers to investigate this process with enhanced precision.
Biophysical methods:
Nuclear Magnetic Resonance (NMR) spectroscopy is a highly effective technique for investigating the composition and movement of α-Synuclein in a liquid state. It can provide comprehensive insights into the structural changes that occur during the aggregation process as well as the interaction between individual units and fibrils. However, the magnitude and intricacy of these formations restrict the use of NMR for investigating substantial aggregates like fibrils.
Cryo-Electron Microscopy (Cryo-EM): The study of protein aggregation has been greatly improved by cryo-electron microscopy (Cryo-EM), which lets scientists see fibrils and oligomers with a level of detail that is almost atomic. This method has yielded crucial observations regarding the arrangement of α-Synuclein fibrils and the processes of secondary nucleation. Recent developments in cryo-electron microscopy (cryo-EM) have made it possible to observe α-Synuclein oligomers. However, this is still difficult because these oligomers are temporary.
X-ray Crystallography: X-ray crystallography, which is typically used to examine the arrangement of highly organized crystals, has also been used to investigate amyloid fibrils. Nevertheless, the use of this method on α-Synuclein oligomers is restricted because of the difficulty in obtaining top-notch crystals of these dynamic and diverse entities.
Spectroscopy and fluorescence techniques are methods used to analyze and study the interaction of light with matter.
Fluorescence Resonance Energy Transfer (FRET) is a commonly employed method for investigating protein-protein interactions. It may also be utilized to observe the aggregation of α-Synuclein in real-time. By tagging α-Synuclein oligomers with fluorescent probes, researchers can track the development of oligomers and fibrils and examine the speed of secondary nucleation.
Thioflavin T (ThT) Assay: A common way to track the growth of amyloid fibrils, like α-Synuclein fibrils, is to use the thioflavin T (ThT) assay. ThT is a fluorescent dye that selectively attaches to the β-sheet structure of fibrils, causing an enhancement in fluorescence. By comparing the rate of fibril formation when preexisting fibrils are present vs absent, this test can investigate the impact of secondary nucleation.
Identifying and describing oligomers is difficult:
Identifying and describing α-Synuclein oligomers continues to be a significant obstacle in the research. Oligomers are temporary, diverse, and exist in small amounts relative to fibrils, rendering them challenging to investigate using traditional methods. New advances in single-molecule techniques, like atomic force microscopy (AFM) and total internal reflection fluorescence (TIRF) microscopy, have made it possible to study oligomers at the level of a single molecule. These approaches provide the necessary sensitivity and precision to identify and analyze oligomers in intricate biological settings.
Therapeutic strategies that aim to inhibit the accumulation of α-Synuclein oligomers:
Secondary nucleation is an important step in the formation of α-Synuclein oligomers. This has opened up new therapeutic possibilities. Various approaches are being investigated to specifically target the aggregation of α-Synuclein and hinder the creation of harmful oligomers.
Inhibitors of Secondary Nucleation: One way to stop secondary nucleation is to make small chemicals that stop α-Synuclein individual units and fibrils from binding, which stops the formation of new oligomers. These inhibitors can be engineered to attach to specific areas of the α-Synuclein molecule or the fibril surface, thereby obstructing the fibrils’ catalytic function. Through high-throughput screening of chemical libraries, several small molecules have been found to effectively stop α-Synuclein oligomers from clumping together in the lab. Currently, these drugs are undergoing evaluation in preclinical models of Parkinson’s disease.
Immunotherapy Targeting Oligomers: Immunotherapy targeting oligomers involves the use of antibodies that are precisely engineered to identify and counteract α-Synuclein oligomers, showing great potential as a treatment method. These antibodies can stop the spread of α-Synuclein disease by attaching to oligomers and making it easier for the immune system to get rid of them. Multiple antibodies targeting α-Synuclein have demonstrated effectiveness in animal models, and a subset of these are presently undergoing evaluation in clinical studies.
Small Molecule Stabilizers: Along with inhibitors and antibodies, small-molecule stabilizers are being looked into as a way to stop α-Synuclein from taking on shapes that make it more likely to clump together. These stabilizers can attach to individual α-Synuclein molecules and hinder the structural alterations that cause the molecules to clump together. These molecules may slow down the formation of both small clusters and long, thread-like structures by keeping the unaggregated form of α-Synuclein stable.
Gene Therapy Approaches: Gene therapy presents an alternative method for specifically addressing the aggregation of α-Synuclein. Gene therapy might be able to directly control the amount of α-Synuclein in the brain by adding genes that make protective proteins or RNA molecules that can lower the expression of α-Synuclein. For instance, RNA interference (RNAi) techniques have been employed to diminish the expression of α-Synuclein in preclinical models, resulting in a reduction in the formation of clumps and the degeneration of nerve cells.
Current Studies and Progress:
A recent study has yielded valuable knowledge about the mechanics of secondary nucleation and its contribution to the aggregation of α-Synuclein. Recent advancements in biophysical techniques have enabled researchers to observe the interaction between individual Researchers can now see a lot more about how individual α-Synuclein monomers and fibrils interact with each other thanks to recent improvements in biophysical techniques. monomers and fibrils with an exceptional level of detail. These investigations have discovered particular areas of the α-Synuclein molecule that are crucial for secondary nucleation and have unveiled the structural characteristics of fibrils that facilitate this process.
In recent years, one important discovery was the identification of different types of α-Synuclein fibrils, which are called polymorphic forms and have their unique properties that affect secondary nucleation. These polymorphs’ varying catalytic abilities in promoting the creation of new oligomers may account for the differences in disease development and observable characteristics across patients with synucleinopathies.
In addition to the structural studies that have already been done, progress in molecular modeling and simulation has opened up new ways of looking at how α-Synuclein assembles. Computational techniques have helped us understand the conformational changes that occur during secondary nucleation and have suggested potential targets for therapeutic intervention.
However, there are still obstacles to converting these discoveries into successful therapies. An important obstacle is the diversity of The diversity of α-Synuclein aggregates in the brain is a significant obstacle. Synuclein aggregates in the brain. Various areas of the brain may have diverse types of α-Synuclein aggregates, each possessing unique characteristics and harmful effects. The presence of heterogeneity makes it more difficult to design medicines that specifically target the aggregation of α-Synuclein. This is because a treatment that is successful in addressing one form of α-Synuclein may not be as effective against another type.
Potential indicators can aid in the early identification of a condition:
The identification of α-Synuclein oligomers as biomarkers for early diagnosis is a subject that is increasingly attracting attention. Because oligomers are early in the illness process, they could be a useful signal for early intervention. Discovering biomarkers that accurately indicate the quantities and functioning of α-Synuclein oligomers in the brain has the potential to facilitate the early detection of synucleinopathies, possibly even before the appearance of clinical symptoms.
Methods for Identifying α-Synuclein Oligomers: Various methods are now under development to identify α-Synuclein oligomers in biological fluids, including cerebrospinal fluid (CSF) and blood. An effective method involves utilizing seed amplification assays, which enhance the quantity of An effective method entails using seed amplification assays, which increase the quantity of α-Synuclein in a sample to levels that can be detected. in a sample to levels that can be detected. The sensitivity and specificity of these assays have been demonstrated in detecting α-Synuclein oligomers. They are currently being assessed as prospective diagnostic tools for Parkinson’s disease and other synucleinopathies.
Implications for Early Intervention: The identification of α-Synuclein oligomers at an early stage could have significant implications for the management of neurodegenerative disorders. oligomers at an early stage could have substantial ramifications for the management of neurodegenerative disorders. By detecting people in the initial phase of the illness, when medical treatments are more likely to have a greater impact, it may be feasible to decelerate or stop the advancement of the condition. Furthermore, the monitoring of α-Synuclein oligomer levels could serve as a method for evaluating the effectiveness of treatment interventions in clinical trials.
Comparative Analysis: α-Synuclein vs Other Amyloid Proteins
Although α-Synuclein possesses unique characteristics, it exhibits some resemblances to other amyloidogenic proteins, including amyloid-β (Aβ) and tau, which are associated with Alzheimer’s disease. Gaining a comprehensive understanding of these similarities and differences can offer vital insights into the underlying processes of protein aggregation and aid in the formulation of effective treatment approaches.
Similarities with Amyloid-β and Tau: Both Amyloid-β (Aβ) and tau have similarities with α-Synuclein in terms of misfolding and aggregation in the brain. This process leads to the formation of hazardous oligomers and fibrils. These proteins also participate in the formation of aggregates within and outside of cells, such as tau tangles and amyloid plaques, which are distinctive features of Alzheimer’s disease. The protein aggregation processes exhibit common characteristics, such as the participation of nucleation, elongation, and secondary nucleation pathways.
Distinctive Characteristics of α-Synuclein Aggregation: Despite these resemblances, α-Synuclein possesses numerous distinct properties that set it apart from other proteins that form amyloid structures. One significant distinction is the prion-like behavior exhibited by α-Synuclein, enabling it to transmit its misfolded state from one cell to another. The prion-like behavior is believed to play a role in the dissemination of α-Synuclein disease throughout the brain in synucleinopathies. However, although there have been suggestions that Aβ and tau have prion-like characteristics, the supporting evidence for this phenomenon is not well-established.
Lessons from Other Protein Aggregation Disorders: Studying the aggregation of α-Synuclein aggregation to other protein aggregation disorders can offer useful insights into the shared processes that cause these diseases. Synuclein about other protein aggregation disorders can offer useful insights into the shared processes that cause these diseases. For instance, the effectiveness of immunotherapy in specifically targeting Aβ in Alzheimer’s disease has served as a source of inspiration for developing comparable methods to target α-Synuclein. Moreover, comprehending the disparities in structure and function among these proteins should provide insight into the varying effectiveness of therapy approaches for different protein aggregation disorders. CSF from Parkinson’s disease patients catalyses oligomer formation.
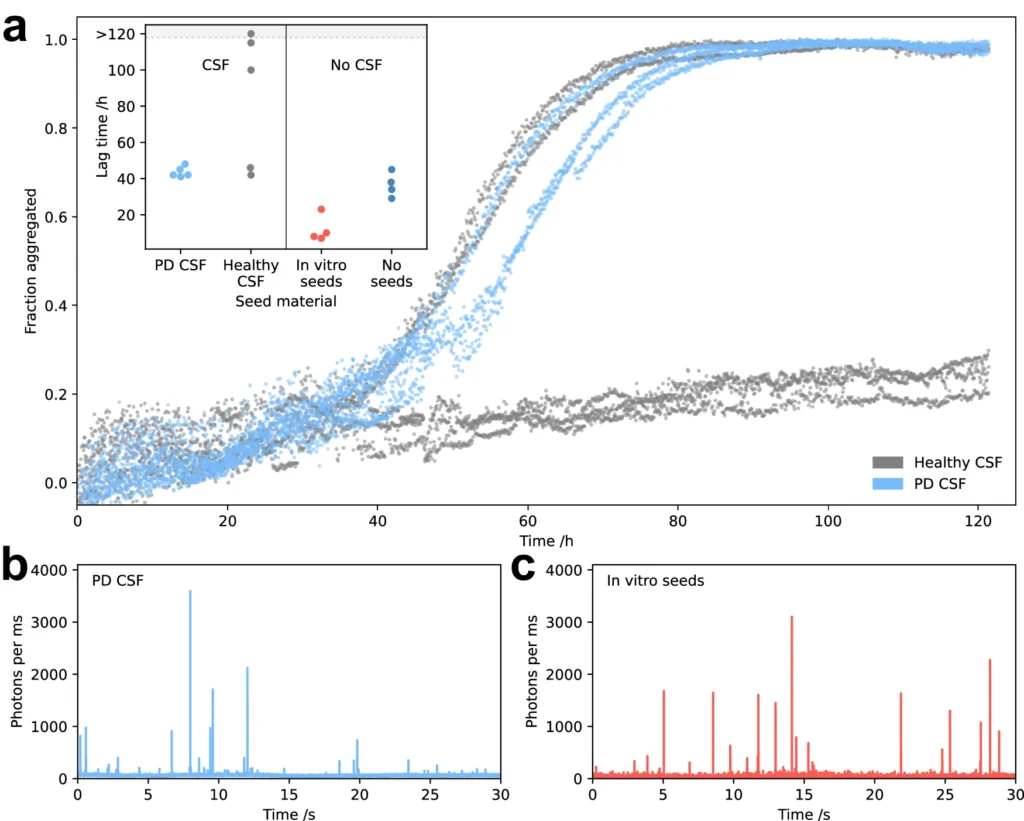
Difficulties and Disputes in the Discipline:
Investigating the process of α-Synuclein aggregation, specifically secondary nucleation, presents various difficulties and areas of disagreement. An ongoing discussion revolves around the comparative significance of oligomers vs fibrils in the development of neurodegenerative disorders. Although oligomers are often considered the most harmful entities, several scientists contend that fibrils and Lewy bodies also have a substantial impact on the advancement of diseases. They either trap toxic oligomers or directly cause damage to neurons.
Technical Limitations in Studying Secondary Nucleation: Technical limits pose a significant problem when examining secondary nucleation. Identifying and describing α-Synuclein oligomers in biological materials is challenging since they are temporary and present in small quantities. As a result, there are inconsistencies in the documented characteristics and harmful effects of oligomers, which have fueled ongoing discussions in the scientific community.
Ethical Considerations in Experimental Models: The utilization of animal models to investigate The use of animal models to investigate α-Synuclein aggregation and neurodegeneration raises ethical concerns about experimental practices. Synuclein aggregation and neurodegeneration give rise to ethical concerns regarding experimental practices. Although these models are extremely useful for comprehending the illness mechanism and evaluating prospective treatments, they also have limits in precisely simulating human diseases. The variations in α-Synuclein expression and aggregation observed between people and animal models may restrict the applicability of research findings. Furthermore, the utilization of animals in scientific investigations requires meticulous adherence to ethical principles to guarantee that studies are carried out with the utmost regard for the well-being of the animals involved.
Future Directions: Despite the obstacles, the α-Synuclein research field is making progress, motivated by the pressing necessity to create successful treatments for neurodegenerative disorders. We expect subsequent investigations to prioritize the resolution of disputes regarding the functions of oligomers and fibrils, the enhancement of methodologies for researching secondary nucleation, and the creation of more precise and morally sound experimental models. Progress in these fields will be crucial for converting our expanding knowledge of α-Synuclein aggregation into successful therapies for synucleinopathies.
In conclusion:
Secondary nucleation plays a crucial role in the development of neurodegenerative illnesses, including Secondary nucleation facilitates the creation of α-Synuclein oligomers, which is critical in the development of neurodegenerative illnesses such as Parkinson’s disease. Parkinson’s disease, by facilitating the creation of α-Synuclein oligomers. The catalytic activity of produced fibrils facilitates the aggregation of α-Synuclein and promotes the propagation of disease in the brain. The oligomers generated during secondary nucleation exhibit significant toxicity and are thought to have a pivotal impact on the advancement of diseases.
Gaining insight into the molecular mechanisms underlying secondary nucleation and identifying the elements that impact this process is essential for the development of therapeutic approaches aimed at targeting the aggregation of Understanding the molecular processes that cause secondary nucleation and figuring out the factors that affect this process is important for creating therapeutic approaches that aim to stop α-Synuclein from clumping together. Recent research breakthroughs have yielded valuable knowledge on the structural and biochemical characteristics of α-Synuclein fibrils and oligomers, identifying possible points of intervention.
Although there has been some progress, there are still difficulties in identifying and describing α-Synuclein oligomers, creating successful treatments, and dealing with the ethical issues related to experimental models. Nevertheless, the persistent efforts of researchers in this domain offer hope for the creation of innovative therapies that have the potential to decelerate or stop the advancement of synucleinopathies, thereby improving the quality of life for people impacted by these debilitating conditions.
Frequently Asked Questions:
1). What is the importance of secondary nucleation in the process of α-Synuclein aggregation?
Secondary nucleation is essential as it expedites the creation of harmful oligomers, which play a critical role in the progression of neurodegeneration. This phenomenon takes place on the outer layer of preexisting fibrils and plays a crucial role in the swift advancement of synucleinopathies.
2). What is the role of What role do α-Synuclein oligomers play in Parkinson’s disease development? oligomers in the development of Parkinson’s disease?
Synuclein oligomers exhibit significant toxicity and can interfere with cellular processes by creating openings in cell membranes, resulting in the entry of calcium ions and subsequent cell death. This mechanism plays a role in the degeneration of dopaminergic neurons in the substantia nigra, which is a characteristic feature of Parkinson’s disease.
3). Is it possible to therapeutically target secondary nucleation?
Indeed, scientists are currently investigating many therapeutic methods to specifically address secondary nucleation. These methods include the use of tiny compounds that hinder the interaction between monomers and fibrils, immunotherapy that targets oligomers, and gene therapy techniques. These techniques aim to reduce the production of harmful oligomers while delaying disease progression.
4). What are the difficulties in identifying α-Synuclein oligomers?
Oligomers are temporary, diverse, and exist in small amounts relative to fibrils, which poses challenges in their detection and research using standard methods. Progress in single-molecule techniques and seed amplification assays is helping to resolve these difficulties.
5). Are there any authorized therapies specifically designed to target the process of Are there any authorized therapies specifically designed to target the α-Synuclein aggregation process? aggregation?
At now, there are no authorized medicines that particularly focus on At present, there are no authorized medicines that specifically target α-Synuclein aggregation. Synuclein aggregation. However, there are other therapies under development, such as small molecule inhibitors, antibodies, and gene therapies. These methods are now undergoing testing in both preclinical and clinical trials to assess their effectiveness in treating synucleinopathies.
For more chemistry blogs, visit chemistry Master