Table of Contents
Overview of 2D Carbon Dot Nanoreactors:
Nanotechnology has produced a diverse array of materials with remarkable catalytic capabilities, among which carbon dots (CDs) have attracted considerable interest. What are 2D Carbon Dot? These are diminutive, carbon-based nanomaterials, generally measuring less than 10 nm, recognized for their remarkable optical, electrical, and chemical characteristics. In recent years, researchers have investigated the possible applications of these dots across diverse domains, including bioimaging and catalysis. Carbon-dot nanoreactors have become popular in catalysis due to their ability to create a regulated microenvironment for reactions, thereby improving efficiency.
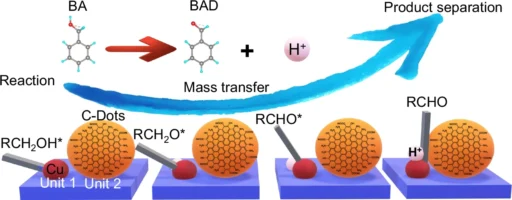
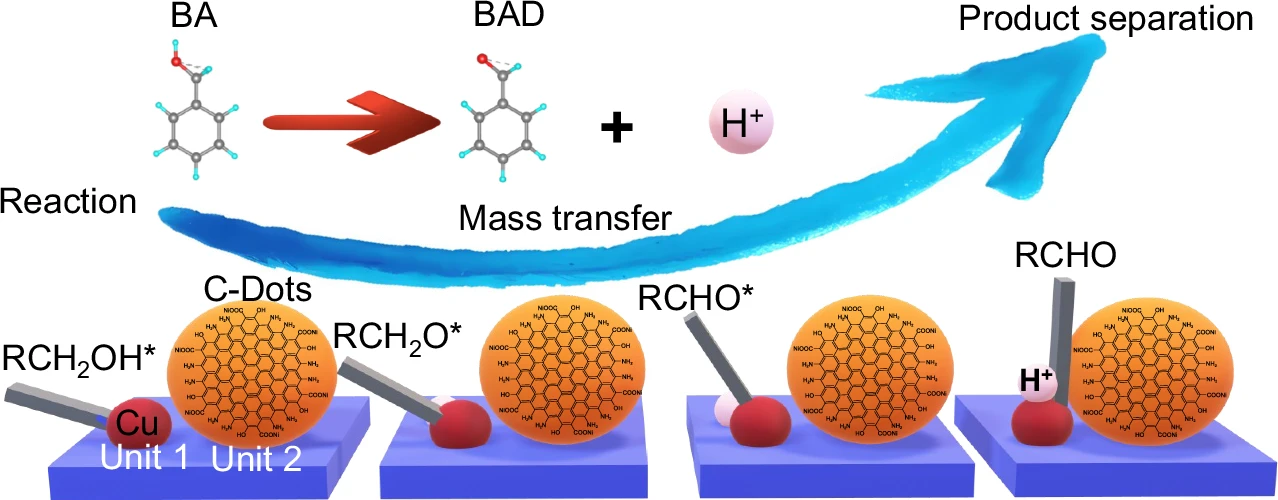
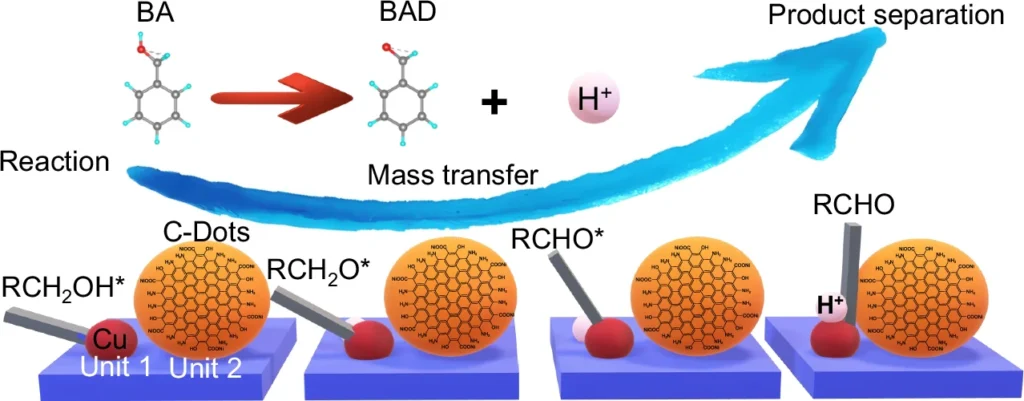
This paper looks at how to make 2D carbon dot nanoreactors that can speed up the oxidation of alcohols and allow hydrogen to be produced at the same time. This is an important process for clean energy and sustainable chemistry. Reaction, mass transfer, and product separation of Cu-ZIS/C-Dots.
Oxidation of Alcohols and Evolution of Hydrogen Reactions:
Before diving into the specifics of carbon dot nanoreactors, it’s crucial to understand the reactions that are being studied: The process involves the oxidation of alcohol and the subsequent evolution of hydrogen.
Alcohol Oxidation: This process involves converting alcohols into aldehydes or ketones, which are essential intermediates in the chemical industry. This procedure conventionally employs hazardous reagents and produces considerable waste, prompting researchers to pursue more sustainable alternatives.
The Hydrogen Evolution Reaction (HER) refers to the generation of hydrogen gas from water, a clean fuel with significant potential in renewable energy systems. Efficient hydrogen production is a problem that requires effective catalysts.
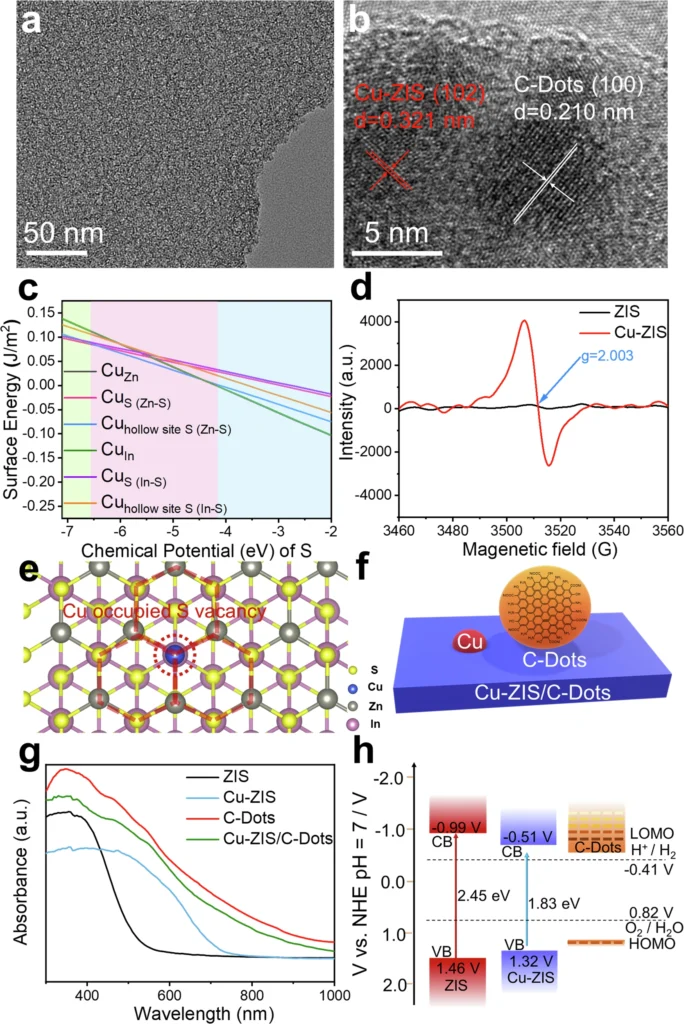
When these two reactions combine, the hydrogen evolution reaction (HER) receives electrons from the oxidation of alcohol, creating a system where both reactions enhance each other. This not only boosts energy efficiency but also yields valuable byproducts. Structure characterizations of Cu-ZIS/C-Dots.
What are the advantages of utilizing 2D carbon dots in catalysis?
What is the rationale for utilizing 2D carbon dots? In comparison to bulk materials or even three-dimensional structures, two-dimensional carbon dots have numerous advantages:
Extensive Surface Area: Their two-dimensional configuration offers a vast surface area, essential for reactions involving several reactants.
Quantum Confinement Effects: Because 2D Carbon Dot are so small, they have unique electronic properties that make them very good at helping electrons move around during redox reactions.
Surface Functional Groups: You can easily functionalize carbon dots with a variety of chemical groups to enhance their catalytic activity, stability, and selectivity.
These attributes render them very suitable for the construction of nanoreactors, particularly for intricate reactions such as alcohol oxidation and hydrogen evolution reactions (HER).
Design Principles for Carbon Dot Nanoreactors:
Designing a carbon dot nanoreactor necessitates meticulous consideration of several parameters to optimize its catalytic effectiveness. These encompass:
Surface Area and Porosity: A larger surface area improves the accessibility of catalytic sites, but regulated porosity allows reactants to access them more efficiently.
Electron Transfer Efficiency: The more rapid the electron transfer between the alcohol oxidation and hydrogen evolution reaction (HER) processes, the greater the overall system efficiency. It is critical to develop nanoreactors to improve transmission.
Functional Grouping: The incorporation of dopants such as nitrogen (N) or sulfur (S) can alter the electrical characteristics of carbon dots, customizing them for particular processes. Catalytic performances of Cu-ZIS/C-Dots.
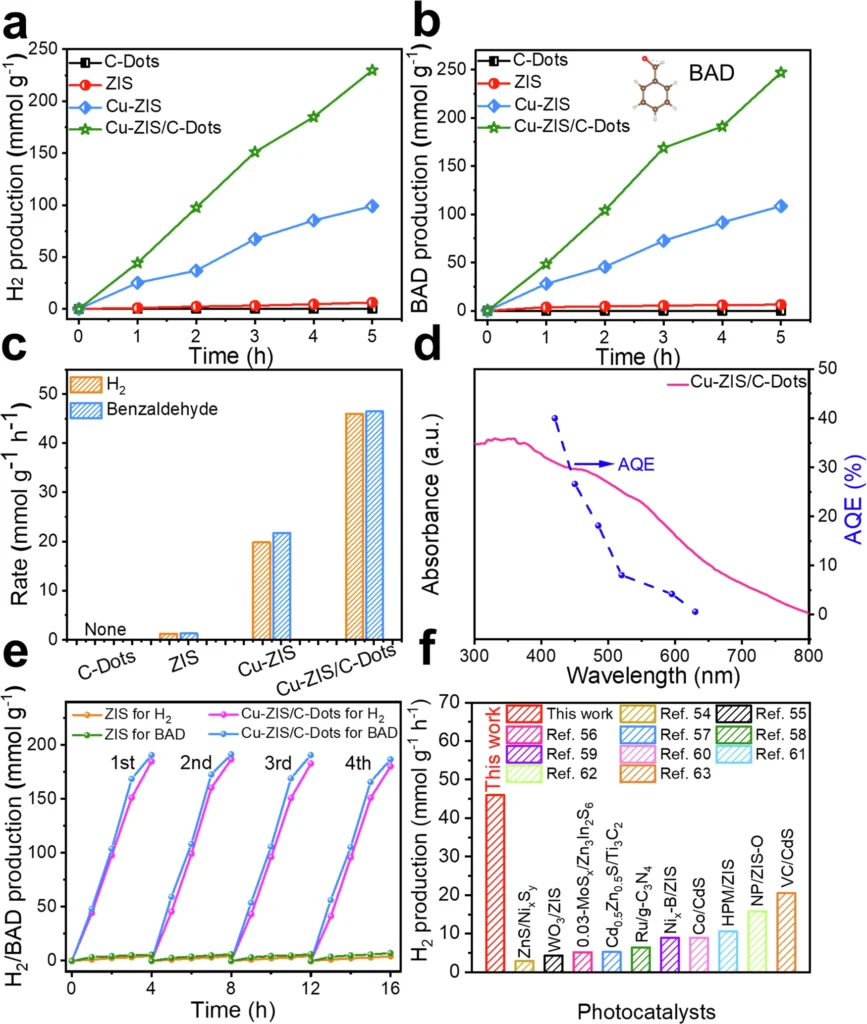
Synthesis of Two-Dimensional Carbon Dot Nanoreactors:
Several methodologies, generally categorized into:
Bottom-Up Synthesis: This entails the fabrication of carbon dots from diminutive organic precursors, typically via pyrolysis or chemical vapor deposition.
Top-Down Synthesis: Conversely, top-down approaches commence with bigger carbon materials (e.g., graphene) and decompose them into nanoscale dots utilizing processes such as laser ablation or chemical exfoliation.
Each method presents unique benefits based on the specific application, although functionalization is paramount. In catalytic applications, the incorporation of elements such as nitrogen or phosphorus can markedly improve performance.
Enhancing 2D carbon dots for alcohol oxidation is the goal:
Careful manipulation can adjust the catalytic activity of carbon dots in alcohol oxidation. Dopants like nitrogen (N), sulfur (S), and phosphorus (P) can generate active sites that facilitate the cleavage of alcohol bonds. Furthermore, regulating the oxidation state and electrical characteristics of carbon dots improves their interaction with alcohol molecules, resulting in increased reaction speeds and selectivity.
Hydrogen Evolution Reaction on Carbon Nanodots:
Her challenge is to optimize the catalytic sites to enhance hydrogen production efficiency. Carbon dots, when functionalized with heteroatoms such as nitrogen or metals like cobalt or platinum, can offer active sites for the hydrogen evolution reaction (HER). Metal-free carbon dots have the potential to be used in hydrogen production, eliminating the need for expensive metals and providing a sustainable alternative.
Integrating Alcohol Oxidation with Hydrogen Production:
Integrating these two reactions necessitates meticulous evaluation of both thermodynamics and kinetics. The oxidation of alcohols generates electrons that can directly power the hydrogen evolution reaction (HER), establishing a synergy that enhances the efficiency of both processes. This not only diminishes energy consumption but also streamlines the reaction setup, rendering it exceptionally appealing for industrial applications. Charge extraction and active species mechanism.
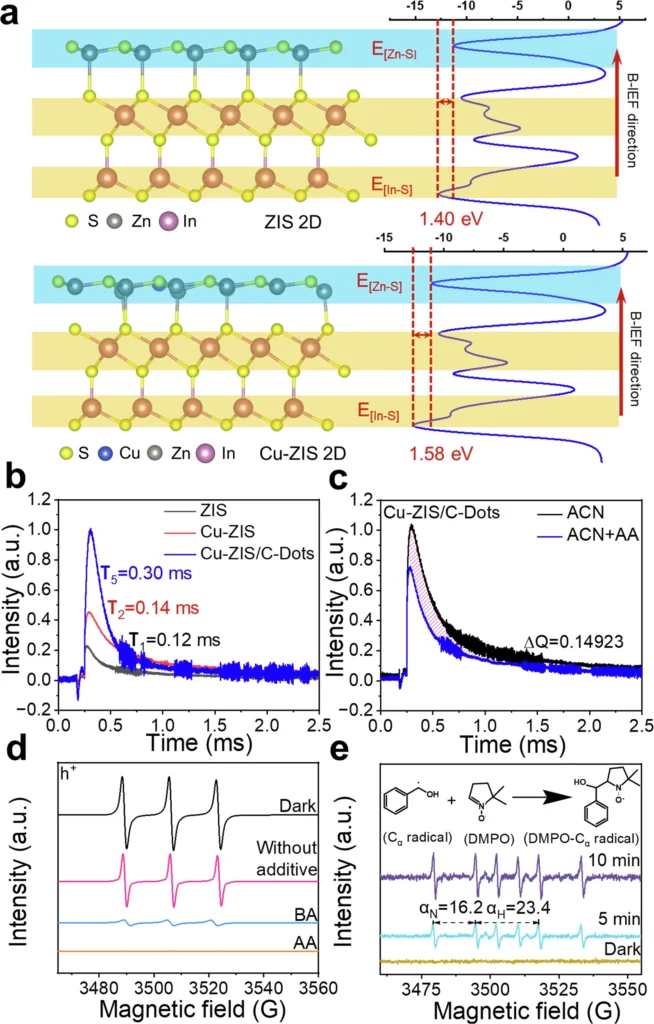
Benefits of Nanoreactors in Coupling Reactions:
Nanoreactors provide a regulated microenvironment that facilitates concurrent reactions, thereby improving both reaction rates and selectivity. Because the nanoreactor is so small, the reactants are guaranteed to be close to each other. This makes it easy for electrons to move quickly between the oxidation and hydrogen evolution reactions. Furthermore, the use of 2D carbon dots reduces energy dissipation, improving the process’s overall efficiency.
The design of 2D carbon dot nanoreactors faces several obstacles:
Despite the benefits, there are still obstacles in the design of stable and efficient carbon dot nanoreactors.
Stability: Carbon dots must preserve their structural integrity throughout reactions, particularly under extreme circumstances.
Deactivation: Catalysts may experience a decrease in activity due to poisoning or aggregation, necessitating design improvements.
Scalability: Transitioning from laboratory-scale synthesis to industrial-scale manufacturing is a considerable challenge, especially in preserving the uniformity of the material properties of carbon dots.
Recent Developments in Carbon Dot Nanoreactor Design:
Recent years have witnessed substantial advancements in the performance of carbon dot nanoreactors. Researchers have created hybrid nanoreactors that integrate carbon dots with various nanomaterials, including metals or metal oxides, to enhance catalytic performance. These hybrid systems facilitate multi-step reactions, creating new opportunities for intricate chemical processes.
Utilizations of Two-Dimensional Carbon Dot Nanoreactors:
The potential applications of 2D carbon dot nanoreactors are vast. In the chemical industry, they may oxidize alcohols into valuable compounds while concurrently generating hydrogen—a clean fuel. Furthermore, environmental operations like water splitting and carbon dioxide reduction can utilize these nanoreactors, contributing to sustainable energy solutions. Mass transfer and product separation.
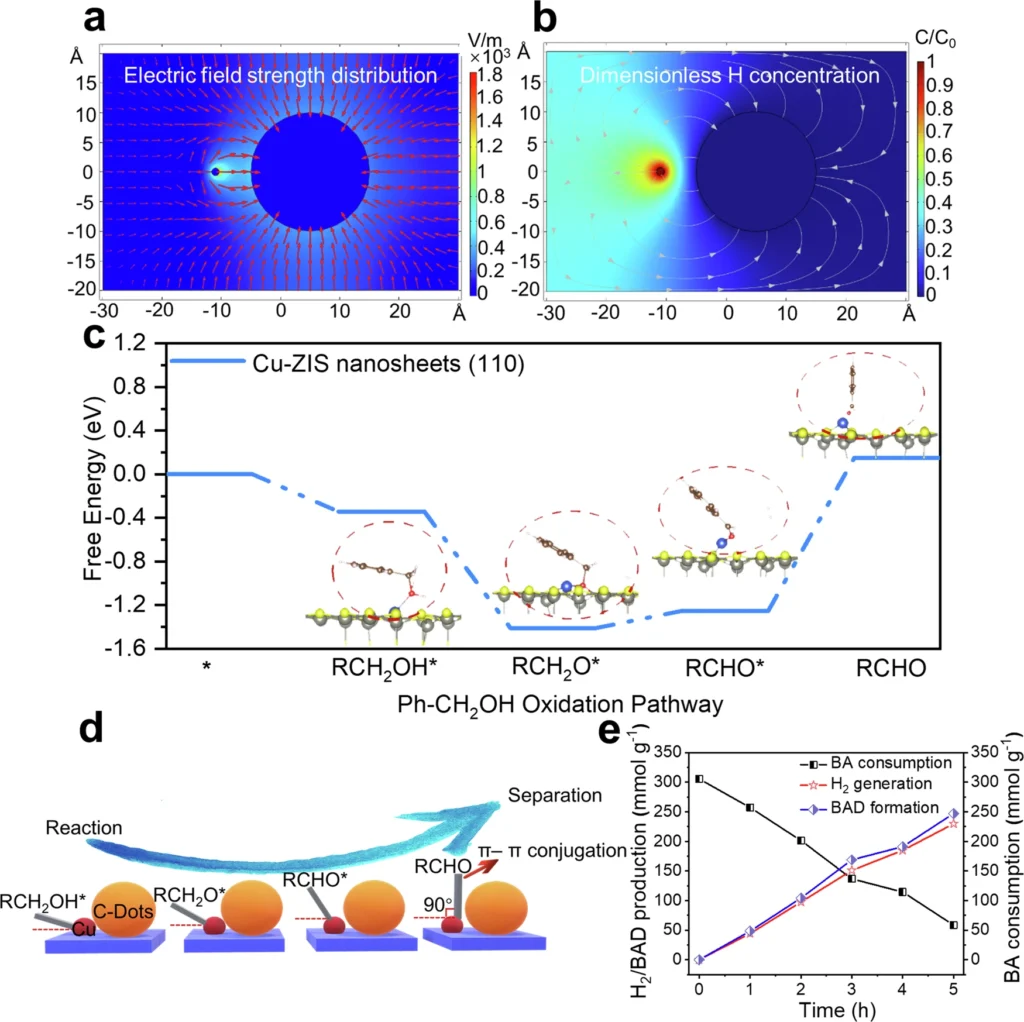
Ecological and financial consequences:
Carbon dots, produced from plentiful and frequently discarded materials, offer a more sustainable alternative to conventional catalysts that rely on scarce or hazardous metals. Their reduced cost, along with their superior efficiency, establishes them as a pivotal participant in the transition to more sustainable industrial practices.
Prospective Developments in Nanoreactor Design:
Future advancements in carbon dot nanoreactors may concentrate on enhancing their stability and scalability as research continues. The integration of carbon dots with renewable energy systems, such as solar-driven catalysis, is a promising area for investigation. We expect researchers to explore additional linked reactions, which could potentially open up new avenues for efficient chemical production.
Final Assessment:
The use of 2D carbon dot nanoreactors for the integration of alcohol oxidation and hydrogen evolution represents a significant advancement in catalysis. Researchers have used the distinctive characteristics of carbon dots to develop a system that is efficient and sustainable, potentially transforming sectors from energy production to chemical synthesis. These nanoreactors may play a pivotal role in the shift towards cleaner, more efficient chemical methodologies as the discipline advances.
Frequently Asked Questions:
1). What are the primary advantages of 2D carbon dots in catalysis?
Two-dimensional carbon dots have a lot of surface area, electrical properties that can be changed, and a functionalization potential that can be set. This makes them very useful for catalysis.
2). What distinguishes carbon dots from other nanomaterials?
Carbon dots are diminutive, possess distinctive optical characteristics, and may be synthesized from many carbon-based substances, in contrast to metal nanoparticles or alternative nanostructures.
3). Can we use 2D carbon-dot nanoreactors for alternative reactions?
These nanoreactors are not only useful for alcohol oxidation and the hydrogen evolution reaction (HER), but also for various processes such as carbon dioxide reduction and water splitting.
4). What are the obstacles to large-scale carbon-dot nanoreactor production?
The challenges include consistency in material qualities, catalyst stability preservation, and economical large-scale production.
5). What is the sustainability of carbon dots in industrial applications?
Carbon dots exhibit significant sustainability, particularly when sourced from waste materials, providing an eco-friendly alternative to conventional metal-based catalysts.
For more chemistry blogs, visit chemistry Master