Table of Contents
Overview:
Sodium-ion batteries (SIBs) have emerged as a possible alternative to lithium-ion batteries (LIBs) in the pursuit of efficient and sustainable energy storage. Due to the plentiful supply and reduced expense of sodium in comparison to lithium, sodium-ion batteries (SIBs) present a practical alternative to meet the increasing need for energy storage devices. Nevertheless, a significant obstacle to the advancement of SIBs is the anode material, which has a direct impact on the total effectiveness of the battery. The 3D combination of molybdenum disulphide (MoS2) and graphene oxide (GO) has attracted considerable interest as a promising anode material for sodium-ion batteries (SIBs).
Comprehension of sodium-ion batteries:
Sodium-ion batteries function similarly to lithium-ion batteries, except that they use sodium ions as electric charge carriers. The batteries consist of a cathode, an anode, an electrolyte, and a separator. During the discharge process, sodium ions migrate from the anode to the cathode, resulting in the production of an electric current. During the charging process, the opposite happens. Although SIBs share some structural characteristics with LIBs, they encounter distinct difficulties because of the bigger dimensions and greater reactivity of sodium ions. Consequently, it is essential to create specialised materials for the anode to attain the best possible performance.
Limitations of Conventional Anode Materials:
Finding appropriate anode materials for SIBs has proven to be difficult. Graphite, a common anode material in lithium-ion batteries (LIBs), lacks efficiency in sodium-ion batteries (SIBs) due to its inability to adequately accommodate sodium ions. This lack of efficiency results in reduced capacity and a restricted lifespan. While hard carbon is an alternative option, it demonstrates superior performance but falls short in terms of energy density and long-term stability. These issues emphasise the necessity for inventive anode materials capable of satisfying the requirements of high-capacity, long-lasting, and secure sodium-ion batteries.
The following factors make 3D MoS2 a promising material for anodes:
Researchers are recognizing molybdenum disulphide (MoS2), a type of layered transition metal dichalcogenide, as a highly promising material for anodes in sodium-ion batteries (SIBs). The distinctive layered structure of MoS2 enables the insertion of sodium ions, resulting in a significantly large theoretical capacity. In addition, its planar structure provides a substantial surface area, thereby promoting electrochemical processes. Nevertheless, MoS2 experiences substantial volumetric growth while undergoing a cycle, resulting in structural deterioration and a swift reduction in capacity. As a result of this restriction, there has been a surge in studies on composite materials that can enhance the stability of MoS2’s structure and its electrochemical capabilities.
Improving Anode Performance via Graphene Oxide:
Graphene oxide (GO) is a compound derived from graphene that possesses exceptional properties such as high electrical conductivity, mechanical strength, and flexibility. Combining graphene oxide (GO) with molybdenum disulphide (MoS2) alleviates the negative aspects of MoS2, such as increased volume and low conductivity. By mixing molybdenum disulphide (MoS2) with graphene oxide (GO), a new material is made that benefits from the best qualities of both. The strong and flexible matrix formed by GO helps to mitigate MoS2 volume variations during cycling. Additionally, it facilitates electron transport by creating conductive routes, thereby improving the overall performance of the anode.
The 3D MoS2/Graphene Oxide Composite: A Revolutionary Advancement
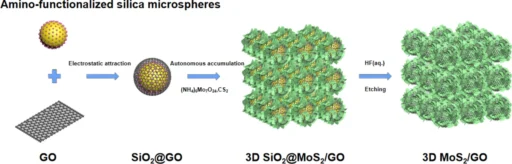
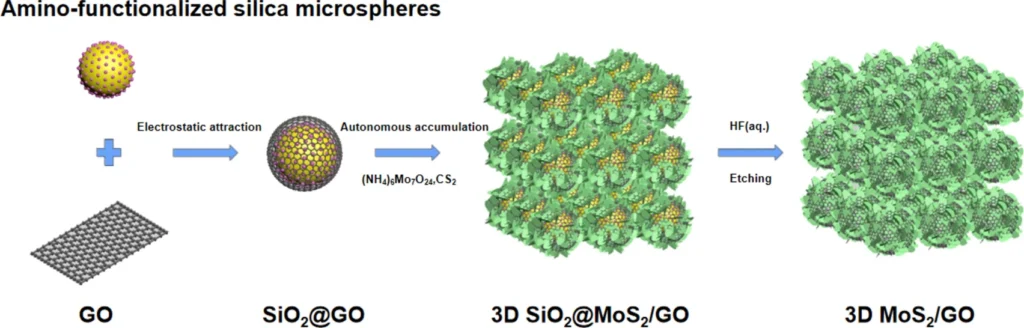
The creation of a composite material consisting of three-dimensional (3D) MoS2 and graphene oxide is a notable progress in the field of anode materials for sodium-ion batteries (SIBs). The 3D architecture has numerous benefits compared to two-dimensional materials, such as increased surface area, enhanced mechanical stability, and more effective ion transport pathways. We commonly use methods like hydrothermal techniques or chemical vapour deposition (CVD) to produce these composites, which result in a porous, interconnected network that promotes the fast diffusion of sodium ions and the transfer of electrons.
The study delves into the electrochemical characteristics of the composite material made up of three-dimensional 3D MoS2 and graphene oxide:
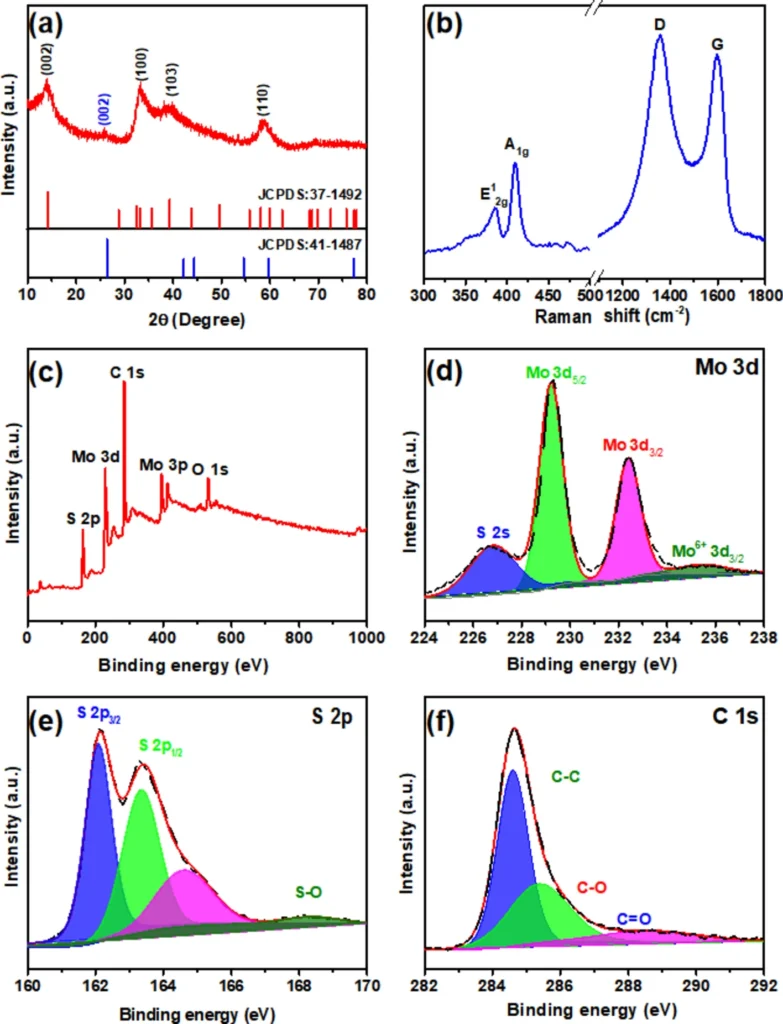
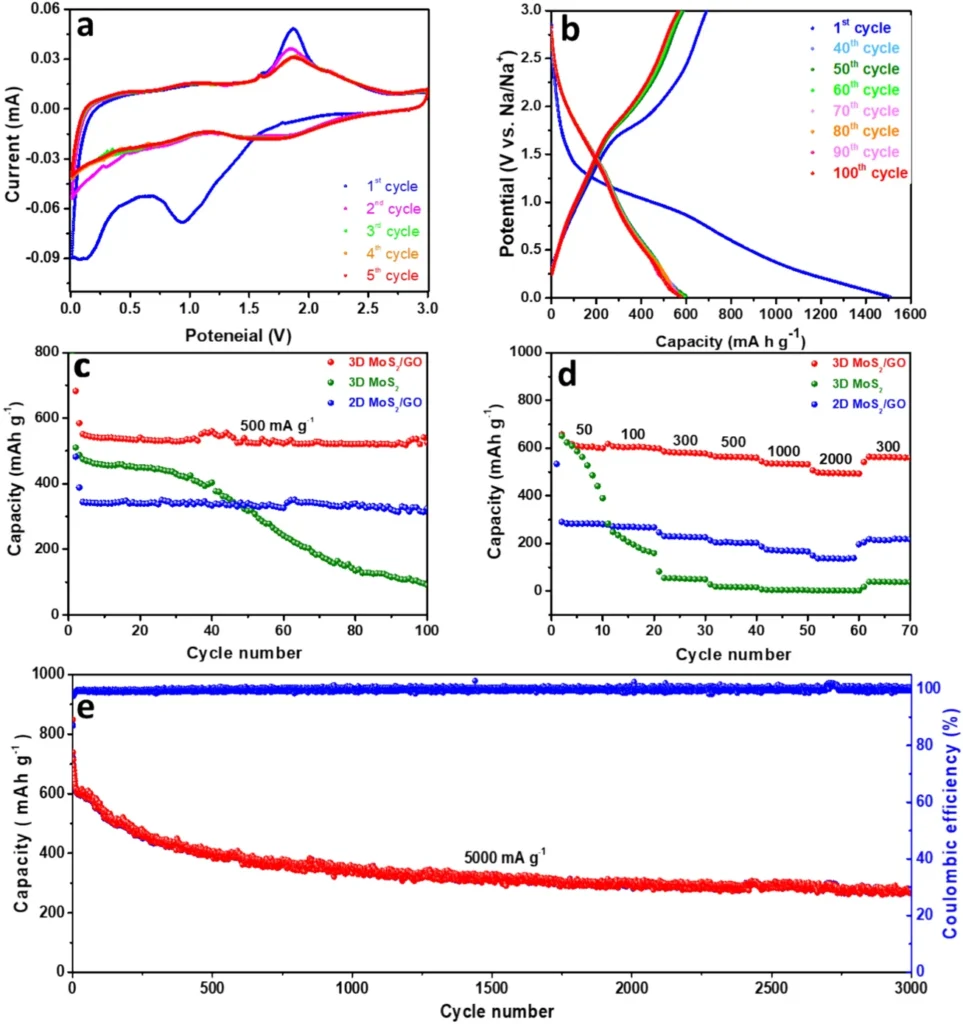
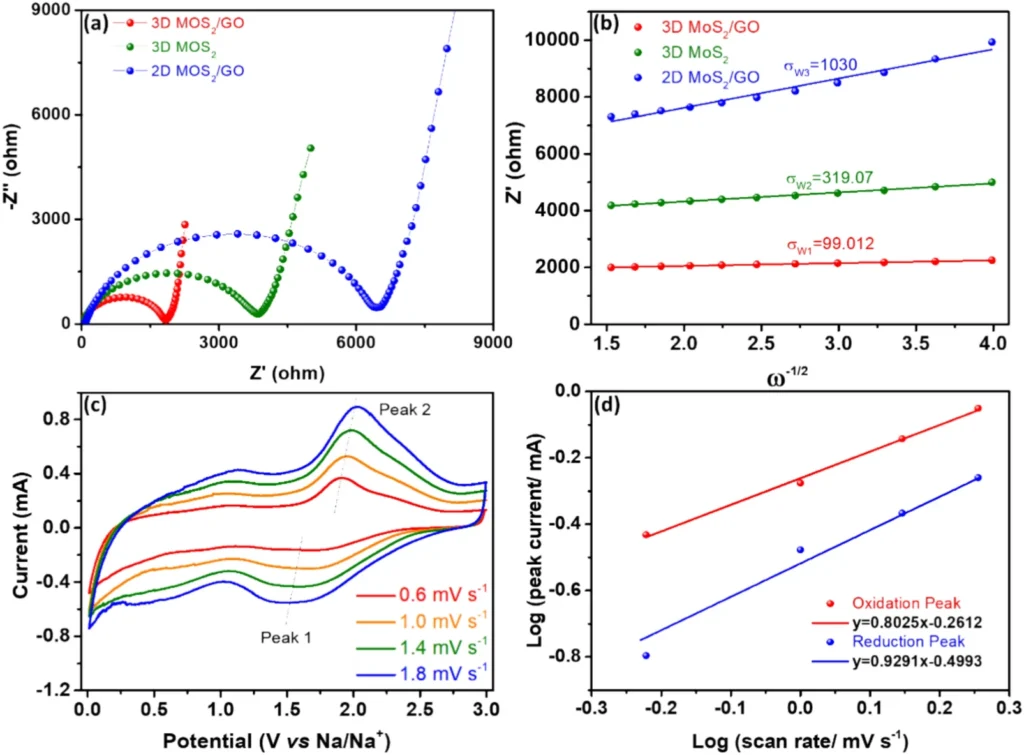
Experiments investigating the electrochemical performance of composites consisting of 3D MoS2 and graphene oxide have shown promising findings. These composite materials generally demonstrate a high specific capacity, exceptional rate capability, and long-term cycling stability. The 3D composite works better than regular anode materials like pure 3D MoS2 or graphene oxide by itself. This is because the 3D design’s beneficial structural features work better with the effects of the individual parts. Furthermore, the composite’s capacity to sustain structural integrity throughout numerous charge-discharge cycles guarantees an extended battery lifespan.
Explanation of the Factors Contributing to Improved Performance:
Various variables contribute to the improved efficiency of the 3D MoS2/graphene oxide composite. Initially, the 3D configuration offers a substantial surface area, increasing the number of active sites available for the sodium ion intercalation process. Furthermore, the inclusion of graphene oxide helps to mitigate the increase in the volume of MoS2, thereby preventing any deterioration in its structure. Furthermore, the interlinked network present in the 3D composite enhances the conductivity of both electrons and ions, resulting in accelerated rates of charging and discharging. Together, the combination of these elements contributes to the exceptional electrochemical performance reported in these composites.
Methods for creating 3D composites of MoS2 and graphene oxide:
Researchers have devised multiple production processes to create composites of 3D MoS2 and graphene oxide, each offering distinct benefits. We extensively use chemical vapour deposition (CVD) due to its capacity to generate materials with high purity and well-defined structures. Hydrothermal synthesis is a commonly used process that enables the creation of composites with a precisely regulated shape and evenly distributed components. We use the layer-by-layer assembly technique to precisely manipulate the composite material’s structure at the nanoscale, ensuring the attainment of ideal performance characteristics. Each of these techniques results in the creation of an efficient composite material that can scale up for industrial applications.
Other Potential Uses for Sodium-Ion Batteries:
Although their applications are not exclusive to this field, 3D MoS2/graphene oxide composites show significant potential as anode materials for SIBs. Alternative energy storage systems like lithium-ion batteries, which face similar challenges with anode materials, can also utilize these materials. Moreover, their distinctive characteristics render them well-suited for application in supercapacitors, solar cells, and even in catalysis for chemical reactions. These composites’ adaptability presents opportunities for further investigation and application in a variety of domains.
We must consider environmental and economic factors:
The sustainability of MoS2 and graphene oxide production is a critical determinant in the widespread acceptance and use of these materials. Nature readily provides Molybdenum disulphide (MoS2) and synthesises it with considerable ease, making it a sustainable choice. However, the expansion of graphene oxide manufacturing requires energy-intensive procedures that could potentially harm the environment. Furthermore, we consider economic factors, ensuring that the cost of producing high-quality 3D composites matches that of existing materials. Conducting life cycle assessments and recyclability studies is essential for gaining a comprehensive understanding of the environmental consequences and economic feasibility of these composites.
Obstacles and Restrictions:
Despite the promising results, there are still obstacles to overcome before introducing 3D MoS2/graphene oxide composites into the market for use in SIBs. Increasing production capacity while ensuring the preservation of material quality is a considerable challenge. Furthermore, it is imperative to conduct comprehensive testing to verify that these composites adhere to industry requirements in terms of their long-term safety and reliability. To tackle these issues, it is necessary to persist in researching and developing new ideas in the fields of material science and engineering.
The latest progress and breakthroughs:
Current studies have focused on improving the synthesis methods and enhancing the efficiency of 3D MoS2/graphene oxide composites. The use of nanostructuring techniques, such as the development of hierarchical porous structures, has shown promise in improving the electrochemical characteristics of these materials. Furthermore, progress in computational modelling is aiding in the comprehension of the underlying mechanics, hence assisting in the development of advanced anode materials for future generations. As research advances, we may anticipate additional enhancements in the performance and versatility of these composites in SIBs and other areas.
In conclusion:
The incorporation of 3D MoS2 and graphene oxide into a composite anode material represents a significant advancement in the development of high-efficiency sodium-ion batteries. This composite combines the positive qualities of both materials and the benefits of a 3D structure to provide improved electrochemical performance, durability, and the possibility for expansion. 3D MoS2/graphene oxide composites have the potential to be a significant factor in the future of battery technology as the need for effective and environmentally friendly energy storage solutions increases.
Frequently Asked Questions:
1). What are the distinguishing characteristics of 3D MoS2/graphene oxide composites in the context of SIBs?
The high capacity of MoS2, the conductivity of graphene oxide, and the 3D structure work together to make the storage of sodium ions much better and improve battery performance.
2). What is the performance comparison between sodium-ion batteries and lithium-ion batteries?
SIBs, or sodium-ion batteries, are more readily available and cost-effective than LIBs, or lithium-ion batteries. However, they typically have a lower energy density. Therefore, the development of new anode materials is essential for enhancing their performance.
3). What are the primary obstacles to utilising MoS2 as an anode material?
MoS2 experiences volume expansion and structural degradation during cycling, which can result in a rapid capacity loss if not well-stabilised.
4). Are 3D MoS2/graphene oxide composites applicable in alternative battery systems?
Indeed, they possess potential uses in lithium-ion batteries, supercapacitors, and various other energy storage technologies.
5). What are the ecological consequences of manufacturing these materials?
Although MoS2 is readily available and environmentally friendly, the manufacturing process of graphene oxide can consume a significant amount of energy. It is crucial to take into account sustainable practices and the ability to recycle.
For more chemistry blogs, visit chemistry Master