Table of Contents
Opening statement:
The synthesis of meta-carbonyl phenols and anilines is a fundamental aspect of contemporary research and industrial applications in the expansive field of organic chemistry. These compounds, characterized by carbonyl groups (-CO-) located in the metaposition of phenolic or aniline functionalities, provide a wide range of structural patterns with significant consequences in numerous domains. This article thoroughly explores the complex techniques used in their creation, examines their diverse uses, addresses the difficulties encountered during production, and explains the potential opportunities that await in the future.
Synthesis Methods:
A Traditional Technique Friedel-Crafts acylation:
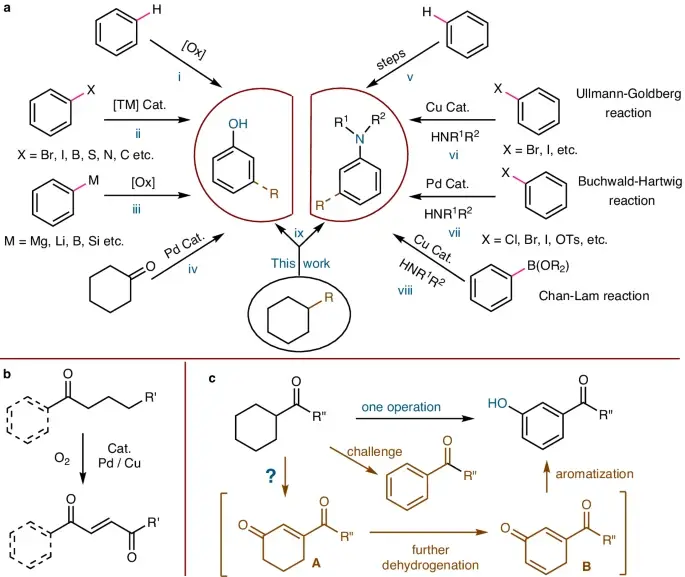
Friedel-Crafts acylation is one of the well-established methods for producing meta-carbonyl phenols. The process described involves introducing an acyl group onto phenolic compounds utilizing acid chlorides or anhydrides in the presence of a Lewis acid catalyst, usually aluminum chloride (AlCl2). We use electrophilic aromatic substitution to insert a carbonyl group at the meta position of the phenolic ring. This process produces the required meta-carbonyl phenol with high selectivity and efficiency.
Ullmann Reaction: Bridging Aromatic Worlds
The Ullmann reaction is a process that connects different aromatic compounds.
The Ullmann reaction is a flexible method for synthesizing meta-carbonyl phenols. It enables the direct arylation of phenolic substrates using aryl halides. When a copper catalyst is used in the right way, this cross-coupling reaction can happen. It does this by making aryl radicals, which then couple to make the desired meta-functionalized products. The Ullmann reaction shows that transition metal catalysis and classical organic synthesis can work well together, making it possible to make meta-carbonyl motifs.
Oxidative Coupling: Broadening Perspectives
Oxidative coupling reactions offer an alternate method for synthesizing meta-carbonyl anilines. This approach allows for the creation of various structural frameworks with exceptional efficiency. We can subject anilinic substrates to oxidative dimerization or oligomerization, resulting in the production of meta-carbonyl anilines, by carefully choosing oxidants and reaction conditions. This innovative approach not only expands the range of synthetic methods available but also opens up new possibilities for exploring uncharted areas of chemistry, thereby enabling the development of new molecular designs and fostering innovations.
Applications:
Pharmaceuticals as Healing Catalysts:
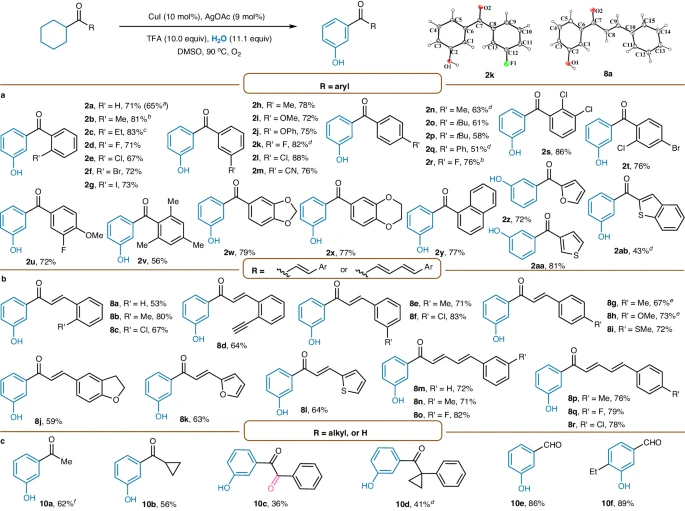
The pharmaceutical industry greatly benefits from the use of meta-carbonyl phenols and anilines due to their ease of synthesis and use in the discovery and development of medical medicines. These compounds have a crucial role as intermediary substances in the production of bioactive molecules, which are essential for the synthesis of medicines, agrochemicals, and therapeutic agents. The deliberate inclusion of carbonyl groups imparts favorable pharmacological characteristics, hence improving the effectiveness and specificity of the produced drugs and promoting progress in the field of medicinal chemistry.
Agrochemicals: Protecting crops
Meta-carbonyl phenols play a crucial role in modern crop protection techniques in the field of agrochemicals. Their strong pesticidal properties make them essential in combating a wide range of agricultural pests and pathogens. Due to their varied structures and ease of synthesis, these compounds play a crucial role in the production of pesticides, herbicides, and fungicides. This helps protect crops and ensures food security worldwide. Meta-carbonyl phenols possess a customized structure that allows for the creation of powerful and specific agrochemicals that have little negative impact on the environment and greatly enhance crop production.
Dyes and pigments are the trailblazers of color:
The dye and pigment industry extensively uses meta-carbonyl anilines due to their exceptional ability to provide vivid colors and a wide range of chromophoric variations. These compounds use their extended π-conjugated systems to display a wide range of colors, from deep blues to vibrant yellows, enhancing the visual appearance of fabrics, plastics, and inks. Meta-carbonyl anilines’ easy manufacturing process enables the creation of customized dyes and pigments, catering to various aesthetic preferences and industrial applications.
Obstacles and resolutions:
Dealing with Selectivity Problems: Understanding and Navigating Regiochemical Landscapes:
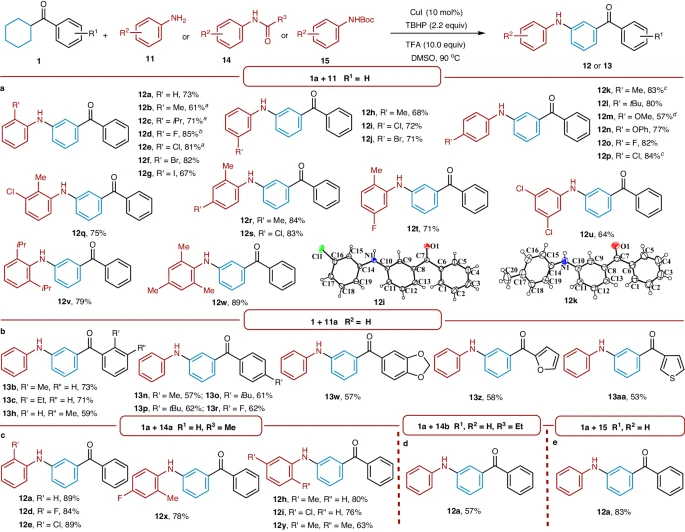
Although there has been significant progress in the synthesis of meta-carbonyl phenols and anilines, there are still challenges in achieving precise control over the location and specific modifications of these compounds. Aromatic substrates naturally react with other molecules, which can lead to ortho- and para-substitution reactions happening at the same time. This creates a wide range of isomeric products. To solve the problem of selectivity, it is necessary to create new catalysts and reaction conditions that can control the specific placement of carbonyl groups. This will make synthetic routes more efficient and increase the amount of product produced.
Catalyst Development: Exploring New Frontiers in Catalysis
The pursuit of effective and environmentally friendly catalytic systems is a new area of focus in the production of meta-carbonyl phenols and anilines. It’s possible for common catalysts used in cross-coupling and oxidative coupling reactions to be less effective, specific, or good at working with functional groups. To get around these problems, we need to create and improve new catalysts, such as transition metal complexes, organocatalysts, and enzymatic catalysts, so that we can make synthesis methods that work well and are good for the environment. Researchers can achieve dramatic breakthroughs in synthetic technique by utilizing the synergistic interplay of catalysis and molecular design, which allows them to explore new areas of chemical space.
Prospects for the Future:
Progress in the development of artificial techniques: expanding the limits
The continuous drive for innovation and exploration drives the ongoing development of synthetic methods for meta-carbonyl phenols and anilines. Novel methodologies like photocatalysis, electrochemistry, and metal-free coupling processes have the potential to significantly transform synthetic pathways and broaden the range of available substrates. Researchers can gain a deeper understanding of chemical reactivity and develop new methods for meta-functionalization by combining experimental and computational approaches. This will lead to advancements in molecular design and synthesis.
Emerging Applications: Accelerating Technological Paradigms
As the need for aromatic compounds with extra functions grows, it becomes clear how important meta-carbonyl phenols and anilines are as catalysts for scientific and industrial progress. Researchers are exploring these compounds for their potential applications in organic electronics, catalysis, and materials science, in addition to their conventional use in medicines, agrochemicals, and dyes. Due to their distinctive electrical and structural characteristics, they are well-suited for use as organic semiconductors, molecular switches, and catalysts. This makes them highly promising for addressing urgent socioeconomic and environmental issues. Researchers can make the most of the natural properties of meta-carbonyl phenols and anilines to help make the future brighter and better for the environment by working together across disciplines and using sustainable chemistry principles.
In conclusion:
Overall, the combination of meta-carbonyl phenols and anilines represents a merging of established methods and new ideas, leading to progress in several fields of science and technology. This article aims to clarify the complex nature of synthetic techniques, explore their various applications, and address the future issues associated with them. Its goal is to shed light on the diverse landscape of meta-functionalization. As we begin our voyage of investigation, let us eagerly welcome the limitless possibilities that lie ahead as we work towards harnessing the revolutionary power of meta-carbonyl phenols and anilines in defining the future of chemistry and beyond.
FAQ (Frequently Asked Questions):
1). What are meta-carbonyl phenols and anilines?
These are chemical compounds called meta-carbonyl phenols and anilines. They have a carbonyl group (C=O) attached to a phenol or aniline molecule at the meta position.
The carbonyl groups (-CO-) in meta-carbonyl phenols and anilines are in the meta position compared to the phenolic or anilinic groups. These are aromatic compounds.
2). What are the main techniques used to produce meta-carbonyl phenols?
The synthesis of meta-carbonyl phenols commonly involves the use of Friedel-Crafts acylation, the Ullmann reaction, and oxidative coupling techniques.
3). What are the key applications of meta-carbonyl phenols and anilines?
These compounds have a wide range of uses in industries such as medicines, agrochemicals, dyes, pigments, organic electronics, and materials science.
4). What challenges are associated with the production of meta-carbonyl phenols and anilines?
Challenges include achieving precise regioselectivity, advancing catalysts, and modifying reaction conditions to improve both efficiency and selectivity.
5). What are the future possibilities for studying this field?
Future research efforts will likely concentrate on enhancing synthetic techniques, creating innovative catalysts, and investigating future applications in organic electronics, catalysis, and sustainable chemistry.
For more chemistry blogs, visit chemistry Master