Table of Contents
An Overview of Trio of Radicals Choreographed:
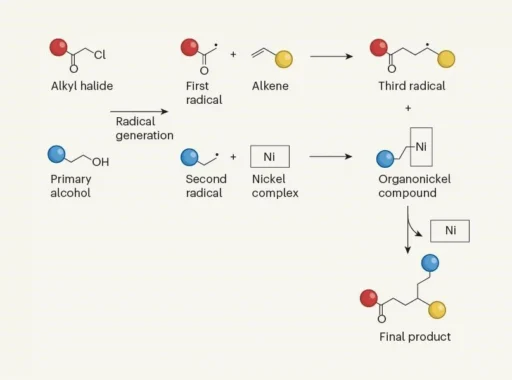
Radical chemistry is an intriguing field of chemical reactions in which extremely reactive particles called radicals carry out complex transformations. The trio of Radicals Choreographed are very reactive because they have unpaired electrons in their molecular structure. This means they can go through a wide range of interesting chemical reactions.
Comprehending the Trio of Radicals Choreographed:
a). What is the definition of radicals?
Radicals are molecular entities with one or more unpaired electrons, making them highly reactive. Their innate reactivity stems from their desire to achieve electron pairing through chemical interactions.
b). What is the radical formation process?
Several processes form radicals, such as the homolytic cleavage of covalent bonds, photolysis, and thermal breakdown. Radical production is commonly caused by ultraviolet (UV) radiation, heat energy, and certain chemical processes.
The Significance of Radical Chemistry:
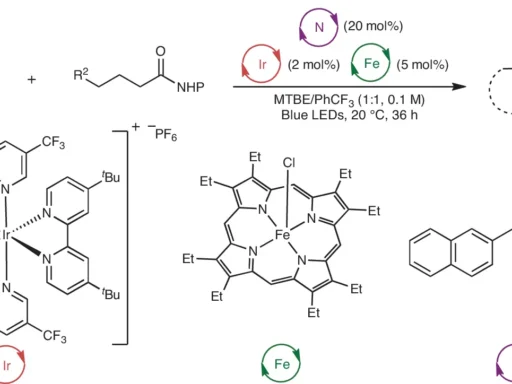
Trio of Radicals Choreographed for Versatile Chemical Reactions is the fundamental basis for a wide range of natural and synthetic processes, and it has a significant impact on various scientific fields. An in-depth understanding of radical reactions enables scientists to develop novel approaches for manipulating molecules and synthesizing materials.
The Triad of Extremists:
Trio of Radicals Choreographed display a wide range of characteristics, but three particular kinds are notable for their commonality and importance in chemical reactions:
a). Radical 1: Hydroxyl Radical (•OH)
The hydroxyl radical, known for its high reactivity and capacity to initiate many chemical reactions, plays a crucial role in atmospheric chemistry, combustion reactions, and the breakdown of contaminants.
b). The radical with the chemical formula CH3 is known as the methyl radical.
Methyl radicals are critical intermediates in various organic reactions, such as combustion, polymerization, and the synthesis of organic molecules through radical-mediated pathways.
c). Nitrogen Dioxide Radical (•NO2) is a type of radical known as Radical 3.
Commonly found in the environment, nitrogen dioxide radicals are essential to atmospheric chemistry, air pollution, and the production of secondary pollutants like ozone.
Creating and directing extreme reactions:
Various methods can be used for trios of Radicals Choreographed for Versatile Chemical Reactions, each designed to support distinct chemical changes:
1). Combustion reactions:
Radicals like hydroxyl and methyl radicals initiate chain reactions during combustion processes, which rapidly transform fuel molecules into heat and light energy.
2). Polymerization reactions:
Radical polymerization adds monomer units one at a time through radical intermediates. This makes polymers with specific properties that can be used in plastics, coatings, and adhesives.
3). Substitution reactions:
Radical substitution reactions allow organic compounds to be modified by substituting one functional group with another, providing synthetic flexibility in the field of organic chemistry.
The Trio of Radicals Choreographed for the versatility of radical reactions:
To put it simply, radical reactions are very useful because they can change many chemical properties, such as creating bonds, breaking bonds, and changing functional groups. The ability to adapt makes radicals extremely valuable tools in synthetic chemistry and materials research.
Utilization of Radical Chemistry:
Many fields use radical chemistry, which has led to advances in:
1). Remediation of the environment:
Radical-mediated activities are crucial in efforts to remediate the environment, as they help break down contaminants and detoxify contaminated areas.
2). Medications:
Radical chemistry plays a crucial role in the creation of pharmaceutical molecules, allowing for the development of intricate molecular structures and the identification of innovative medicinal substances.
3). Study of the properties, structure, and performance of materials:
In the field of materials science, radical reactions are utilized to create and produce functional materials that possess certain features, such as conductive polymers, responsive gels, and molecularly imprinted polymers.
Obstacles and Prospects for the Future :
Despite their usefulness, radical reactions pose hurdles in terms of selectivity, efficiency, and environmental effect. To tackle these issues, it is imperative to create innovative catalysts, develop new reaction techniques, and adopt sustainable practices.
a). Regulation of Extreme Reactivity:
The objective of initiatives focused on limiting radical reactivity is to improve the selectivity and efficiency of reactions while reducing unwanted side reactions and byproducts.
b). Green Chemistry Initiatives:
Efforts to promote sustainable and environmentally friendly practices in the field of chemistry. Green chemistry projects promote the creation of sustainable radical reactions that minimize waste production, decrease energy usage, and utilize renewable resources.
In conclusion:
To summarize, the group of Trio of Radicals Choreographed, radicals consisting of hydroxyl, methyl, and nitrogen dioxide exemplifies the adaptability and importance of radical chemistry. Radical reactions play a crucial role in driving scientific innovation and technological growth across various fields, including environmental remediation and pharmaceutical synthesis.
Frequently Asked Questions:
1). What is the definition of radical reactions?
Radical reactions entail the involvement of extremely reactive molecular species known as radicals. These radicals are distinguished by the presence of unpaired electrons and are capable of undergoing a variety of chemical transformations.
2). What is the radical formation process?
Several mechanisms, such as homolytic breakage of covalent bonds, photolysis, and thermal breakdown, can generate radicals.
3). What are some practical uses of radical chemistry?
Radical chemistry is utilized in several fields, such as environmental remediation, pharmaceutical production, materials research, and chemical synthesis.
4). What difficulties do radical reactions present?
The challenges related to radical reactions encompass the management of reactivity, selectivity, and the reduction of environmental effects.
5). What tactics can we use to improve radical reactions’ sustainability?
By advancing green chemistry techniques, catalysts, and reaction conditions, we can achieve sustainable radical reactions.
For more chemistry blogs, visit chemistry Master