Table of Contents
In the field of organic chemistry, the creation of intricate molecules is crucial for the advancement of drug development, material research, and other scientific pursuits. Benzoacridines and indenoquinolines are notable compounds in the field of medicinal chemistry due to their wide range of pharmacological properties and prospective applications. Researchers have been working to create effective ways to make these chemicals, and recent progress in catalysis has introduced acidic magnetic dendrimers as potential catalysts for their production.
An overview of benzoacridines and indenoquinolines:
Benzoacridines and indenoquinolines are classified as heterocyclic compounds, which are characterized by the fusion of benzene and acridine rings in the case of benzoacridines and indene and quinoline rings in the case of indenoquinolines. These molecules display a diverse array of biological activities, such as their ability to combat cancer, germs, viruses, and inflammation. The wide range of pharmacological characteristics exhibited by these compounds has generated significant enthusiasm for their potential use in drug development and medicinal chemistry research.
The Significance of Effective Synthesis Techniques:
Efficient synthetic techniques are required for the rapid and cost-effective production of benzoacridines and indenoquinolines. Conventional methods for creating synthetic compounds typically require several stages, involve intense reaction conditions, and result in limited overall production, which presents considerable difficulties when it comes to expanding the process, ensuring consistent results, and maintaining environmental friendliness. Hence, it is crucial to prioritize the advancement of effective and environmentally friendly methods for synthesizing these essential molecules to satisfy the increasing need for them.
An Overview of Acidic Magnetic Dendrimers as Catalysts:
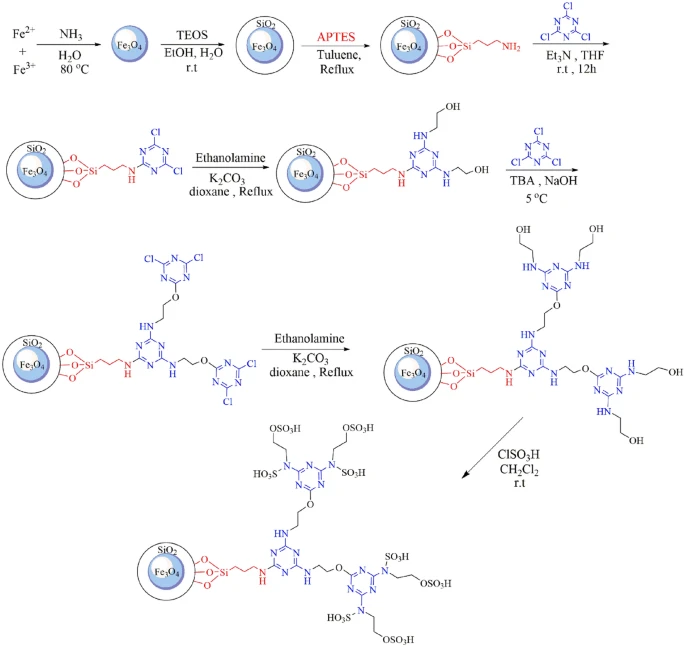
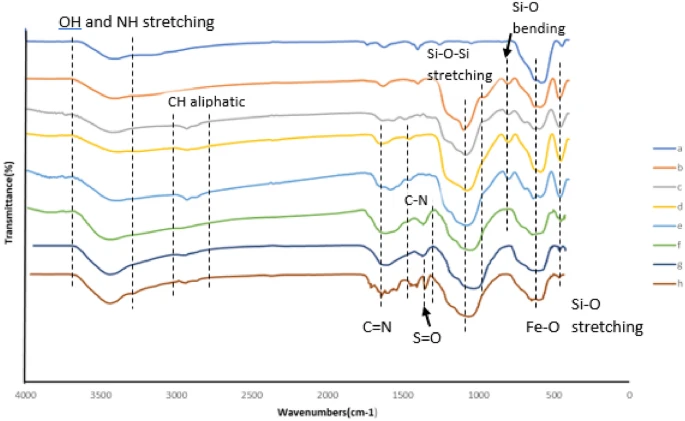
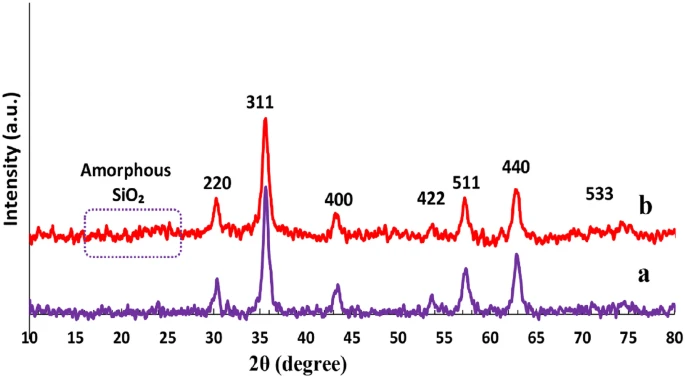
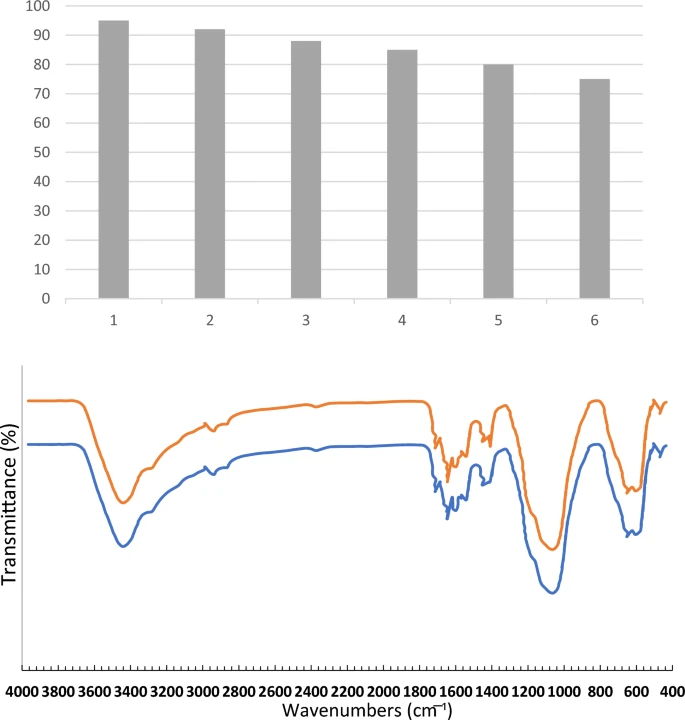
Acidic magnetic dendrimers are a type of nanostructured material that merges the catalytic activity of acidic functional groups with the magnetic properties of nanoparticles. Hybrid materials offer numerous advantages as catalysts, including their large surface area, adjustable acidity, magnetic recovery capacity, and potential for recycling. When you mix dendritic polymers with magnetic nanoparticles, you get a flexible framework that makes chemical reactions easier at low temperatures. This makes them good choices for making complex compounds like benzoacridines and indenoquinolines.
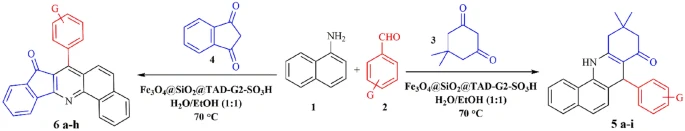
An examination of the process for creating benzoacridines using acidic magnetic dendrimers as catalysts:
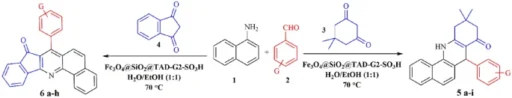
The process of creating benzoacridines using acidic magnetic dendrimers as catalysts usually entails the combination of o-aminobenzaldehydes with aromatic ketones in the presence of the dendrimer catalyst. The presence of acidic sites on the surface of the dendrimer enables the carbonyl group in the ketone to be activated. This activation promotes the nucleophilic addition of the aldehyde’s amino group, resulting in the formation of the desired benzoacridine scaffold. The dendrimer catalyst’s magnetic recoverability facilitates effortless separation and recycling, hence improving the sustainability and practicality of the synthetic process.
Catalytic Reaction Mechanism:
The benzoacridine synthesis process is made up of several steps. 1). The ketone is protonated at the acidic sites of the dendrimer catalyst to make it work. 2). The amino group of the aldehyde then attacks the activated ketone using a nucleophilic attack, and the imine intermediate is cyclized within the molecule through electrophilic aromatic substitution. 3). Dendritic polymers and magnetic nanoparticles work together to increase catalytic turnover efficiency and promote the synthesis of certain products under gentle reaction conditions.
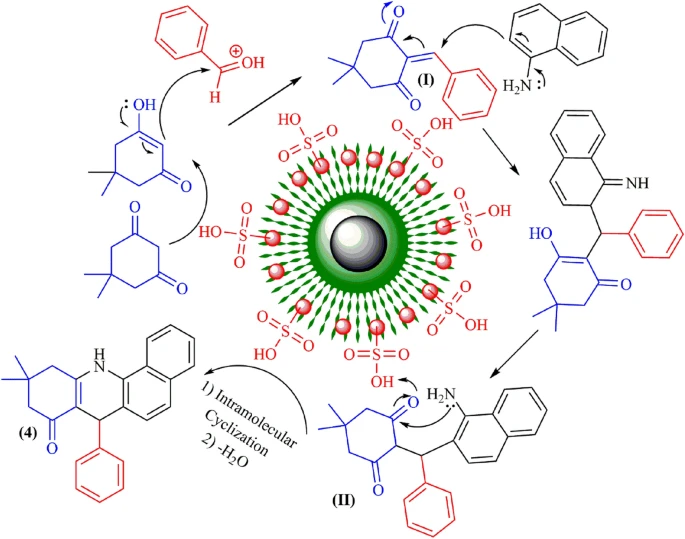
Factors that significantly influence the synthesis process’s efficiency:
The efficiency of benzoacridine synthesis catalyzed by acidic magnetic dendrimers is influenced by various parameters, such as a). catalyst loading, b). reaction temperature, c). solvent selection, d). substrate range. It is crucial to optimize these parameters to get the highest possible conversion and yield while minimizing the occurrence of side reactions and the amount of wasted catalyst. It is possible to change the reaction conditions very precisely to get the best performance and selectivity in the synthesis of benzoacridine because dendrimer catalysts are very flexible and adaptable.
Uses of Benzoacridines and Indenoquinolines:
Pharmaceutical chemistry, materials science, and biological research widely utilize benzoacridines and indenoquinolines due to their unique pharmacological qualities and structural characteristics. They have undergone investigation for their potential utilization as anticancer drugs, antibacterial agents, fluorescent probes, and molecular imaging agents. The development of effective synthetic techniques for these substances opens up new opportunities for pharmaceutical research, molecular imaging, and chemical biology investigations.
An examination of the process for creating indenoquinolines using acidic magnetic dendrimers:
The process of synthesizing indenoquinolines using acidic magnetic dendrimers is based on the same principle as the synthesis of benzoacridine. It is done by mixing 2-aminobenzyl alcohols with α,β-unsaturated ketones, or aldehydes while the dendrimer catalyst is present. The acid sites on the dendrimer’s surface start the reaction of the carbonyl group in the unsaturated ketone or aldehyde, which helps the amino group of the alcohol bind to the dendrimer. This reaction leads to the formation of the indenoquinoline scaffold, as desired. The dendrimer catalyst’s magnetic recoverability facilitates effortless separation and recycling, enhancing the sustainability and cost-effectiveness of the synthetic process.
Comparison to conventional synthesis methods:
The catalytic synthesis of benzoacridines and indenoquinolines utilizing acidic magnetic dendrimers provides various advantages over previous synthesis methods. These advantages include the use of milder reaction conditions, increased selectivity, decreased environmental effects, and improved practicality. The catalyst’s ability to help reactions happen at low temperatures and its magnetic retrievability make the process more efficient and long-lasting. This makes dendrimer-catalyzed synthesis processes very appealing for industrial uses and large-scale production.
Benefits of Using Acidic Magnetic Dendrimers as Catalysts:
Acidic magnetic dendrimers possess numerous intrinsic benefits as catalysts for organic synthesis, including exceptional catalytic activity, adjustable acidity, the ability to be recovered using magnets, the capability to be reused, and compatibility with a broad spectrum of substrates. The combined effects of dendritic polymers and magnetic nanoparticles offer a flexible foundation for catalyzing various chemical transformations, facilitating the creation of efficient and sustainable synthesis techniques for intricate compounds such as benzoacridines and indenoquinolines.
Illustrative Case Studies Highlighting the Synthesis Techniques’ Efficacy:
An abundance of research has shown that acidic magnetic dendrimers are very good at speeding up the production of benzoacridines and indenoquinolines. These case studies demonstrate the ability to scale up, replicate, and apply catalytic synthesis processes in several areas of synthetic applications, such as pharmaceutical chemistry, materials science, and chemical biology. The ability of the catalyst to carry out reactions at low temperatures and its magnetic retrievability make the process more efficient and long-lasting. This makes dendrimer-catalyzed synthesis processes very appealing for industrial uses and large-scale production.
Future Prospects and Advancements in the Field:
Potential opportunities and advancements in the field of study in the coming years.
We expect advancements and improvements in the field of catalytic synthesis employing acidic magnetic dendrimers. Researchers are focusing their current efforts on developing new dendrimer catalysts, comprehending the mechanisms of reactions, broadening the scope of substances they can use, and integrating these catalysts with other systems to achieve various tasks. Researchers are utilizing the distinct characteristics of dendritic polymers and magnetic nanoparticles to tackle existing obstacles in organic synthesis. Their goal is to establish more effective, environmentally friendly, and feasible methods for synthesizing intricate molecules such as benzoacridines and indenoquinolines.
In conclusion:
Acidic magnetic dendrimers are very useful as catalysts for making benzoacridines and indenoquinolines. This is a big step forward in the field of organic synthesis because it works so well. These novel synthesis methods, which use the catalytic capabilities of dendritic polymers and magnetic nanoparticles, provide several benefits compared to conventional procedures. These advantages include gentler reaction conditions, more selectivity, decreased environmental impact, and improved practicality. Acidic magnetic dendrimers have the potential to be adaptable and environmentally friendly catalysts for creating various heterocyclic compounds through ongoing research and advancement. The development of medicine, materials science, and chemical biology could potentially utilize these compounds.
Frequently Asked Questions:
Benzoacridines and indenoquinolines are organic compounds of significant importance, why?
They possess unique chemical structures and exhibit various properties that make them valuable in several fields of study. Understanding these compounds is crucial for advancing research and development in areas such as pharmaceuticals, materials science, and organic synthesis.
Heterocyclic compounds such as benzoacridines and indenoquinolines, exhibiting a wide range of biological activities, hold potential for use in drug research and materials science.
What is the synthesis method for benzoacridines and indenoquinolines using acidic magnetic dendrimers?
Benzoacridines and indenoquinolines can be synthesized using catalytic means utilizing acidic magnetic dendrimers as catalysts. These catalysts enable condensation reactions between suitable substrates to occur under gentle circumstances.
What are the advantages of using acidic magnetic dendrimers as catalysts for synthesis?
Acidic magnetic dendrimers offer a multitude of advantages, including enhanced catalytic efficiency, customizable acidity, magnetic recovery capability, recyclability, and compatibility with a wide range of substrates.
The applications of benzoacridines and indenoquinolines are varied and diverse, why?
Because of their unique pharmacological effects and structural characteristics, medicinal chemistry, materials science, and chemical biology widely use benzoacridines and indenoquinolines.
What are the potential outcomes for the future of synthesis methods catalyzed by dendrimers?
In the future, scientists will study dendrimer-catalyzed synthesis to find new catalysts, better understand how reactions work, use dendrimer catalysis on more complex substrates, and mix dendrimer catalysis with other catalytic systems to make changes that have more than one use.
For more chemistry blogs, visit chemistry Master