Table of Contents
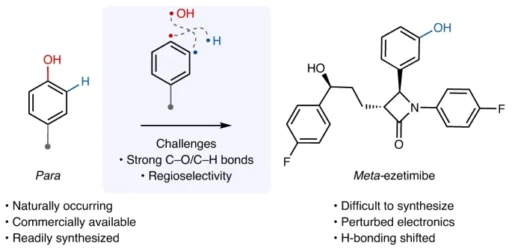
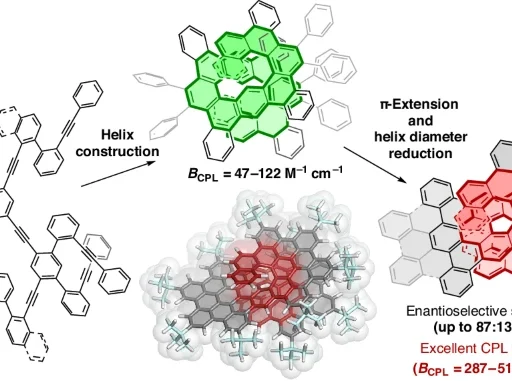
Isomerization of Phenols: Phenols are a type of organic molecule that has a hydroxyl group (-OH) connected to an aromatic ring. They possess interesting chemical features that have captivated chemists for many years. Para-to meta-isomerization of phenol is a notable phenomenon observed in phenolic compounds, with important consequences in organic synthesis and chemical biology.
An Overview of and Isomerization of Phenol:
Phenol: The hydroxyl group directly attaches to an aromatic carbon atom in phenols, which are aromatic compounds. These substances are frequently present in natural products such as essential oils, antioxidants, and medicines. Isomerization is the process of rearranging atoms within a molecule to create isomeric compounds. These molecules have the same chemical formula but distinct structural configurations.
Isomerization: are significant chemical compounds that consist of a hydroxyl group (-OH) directly linked to an aromatic ring. Nature widely distributes these chemicals, including essential oils, antioxidants, and medicines. The fundamental chemical process of isomerization involves the rearranging of atoms within a molecule to produce isomeric compounds. These molecules have the same molecular formula but distinct structural configurations. Isomerization reactions are common in organic chemistry and play an important role in the creation of intricate molecules and the advancement of new materials.
Definition and Mechanism of Isomerization of phenol:
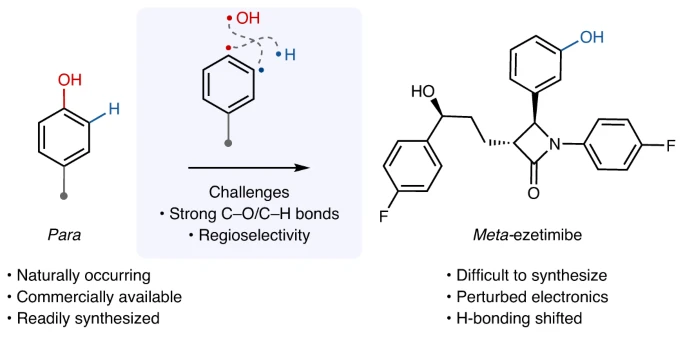
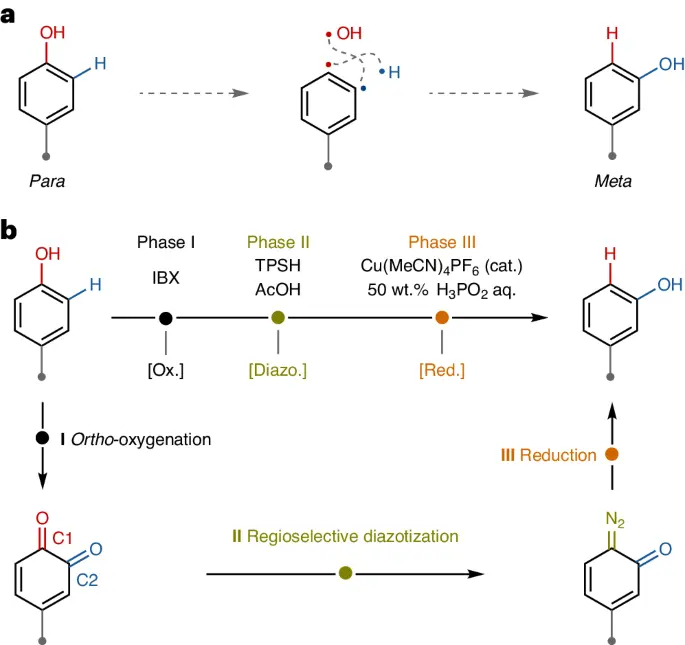
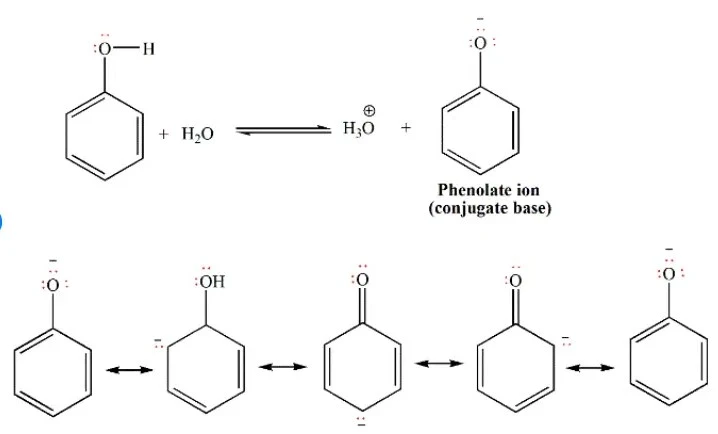
Isomerization of phenol refers to the transformation of a phenol molecule in which the hydroxyl group is situated at the para position concerning a substituent on the aromatic ring into a molecule where the hydroxyl group is positioned at the meta position. Different circumstances, such as temperature, solvent, and the presence of catalysts, can affect this reorganization. Most of the time, the isomerization of phenol happens through electrophilic aromatic substitution reactions, which create and then rearrange carbocation intermediates.
Para-to-meta-isomerization of phenol is a chemical reaction where the atoms in a phenol molecule are rearranged, causing the hydroxyl group to move from the para position to the meta position on the aromatic ring. Parameters such as temperature, solvent polarity, and the presence of catalysts influence this conversion, which can occur through diverse methods. Electrophilic aromatic substitution is what makes the isomerization reaction happen. The aromatic ring acts as a nucleophile and reacts with an electrophilic molecule. This results in the production of a carbohydrate intermediate. The hydroxyl group migrates to the meta position as a result of the carbocation’s subsequent rearrangement.
The significance of isomerization of phenol :
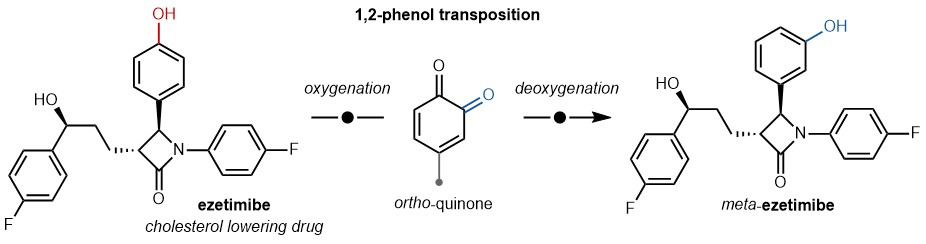
The conversion of phenols from the para- to meta-isomer has important significance in organic synthesis, especially in the creation and advancement of medicines and agrochemicals. Chemists can adjust the characteristics of organic compounds, such as their reactivity, stability, and bioavailability, by changing the arrangement of functional groups on the aromatic ring. Furthermore, the conversion from para- to meta-isomerization of phenol is of utmost importance in various biological processes, such as the breakdown of foreign substances and the triggering of pharmacological effects in the human body.
Para-to-meta-isomerization of phenol is a crucial reaction in organic chemistry that has various uses in the production of medicines, agrochemicals, and other useful materials. Chemists can precisely adjust the properties of organic molecules, such as their reactivity, stability, and biological activity, by strategically altering the position of functional groups on the aromatic ring. This allows for the creation and advancement of new medications that have enhanced effectiveness and fewer adverse reactions. Furthermore, the conversion from para- to meta-isomerization of phenol is of utmost importance in various biological processes, such as the breakdown of medicines and foreign substances in the human body. As a result, understanding the processes and dynamics of isomerization of phenol reactions is critical for the logical formulation of therapeutic drugs as well as the advancement of novel approaches to drug administration and targeting.
Techniques for inducing the conversion of para-isomers to meta-isomerization of phenol:
Various techniques have been devised to trigger the conversion of para- to meta-isomers in phenolic compounds. These processes include thermal reactions, in which heat induces chemical bond reorganization, as well as catalytic reactions assisted by transition metal complexes or acidic environments. Additional methods, such as photochemical isomerization and enzymatic transformations, provide alternate approaches to regulating the isomeric composition of phenolic compounds.
Phenols can undergo para- to meta-isomerization of phenol by many means, such as heat processes, catalytic reactions, and photochemical transformations. The process of thermal isomerization involves heating the phenolic compound to trigger the rearrangement of chemical bonds and the transfer of the hydroxyl group to the meta position. Catalytic isomerization, in contrast, depends on the utilization of transition metal complexes or acidic conditions to assist the rearrangement step. Photochemical isomerization of phenol involves the use of light energy to stimulate the creation of reactive intermediates, which subsequently undergo rearrangement to produce the meta-isomer. Enzymatic transformations use enzymes as catalysts to induce isomerization events in phenolic substances selectively and ecologically friendly.
Illustrations of Para- to Meta-Isomerization of phenol Reactions:
Phenols undergo many chemical processes that showcase the conversion from para- to meta-isomers. These reactions include both straightforward rearrangements catalyzed by acids and more intricate multistep transformations. An example that demonstrates the process of para-to-meta-isomerization is the conversion of hydroquinone to p-benzoquinone in acidic circumstances. Drug discovery has selectively modified phenolic functionalities to create new therapies with enhanced pharmacological properties.
Para- to meta-isomerization of phenol reactions is commonly reported in many chemical transformations, ranging from basic acid-catalyzed rearrangements to intricate multistep reactions. For example, the transformation of hydroquinone to p-benzoquinone in acidic conditions entails the relocation of the hydroxyl group from the para position to the meta position on the aromatic ring. Similarly, scientists have utilized the deliberate alteration of phenolic functions to develop novel medications with enhanced effectiveness and specificity. By changing where the functional groups are placed on the aromatic ring, medicinal chemists can improve the therapeutic drugs’ pharmacological properties and reduce the effects that aren’t intended.
Obstacles and Constraints in Investigating Isomerization of Phenol:
Although para-to-meta-isomerization of phenol is significant, it poses many difficulties for researchers. The challenges encountered in studying the fundamental processes of isomerization include the ability to control the desired outcome of reactions and accurately identify and study the short-lived intermediate species. Furthermore, the intricate nature of phenolic transformations necessitates the use of advanced computational and spectroscopic approaches to understand the complexity of reaction pathways and the impact of solvent effects.
Investigating the process of para-to-meta-isomerization poses many difficulties because of the intricate reaction mechanism and the fleeting existence of intermediate species. The advancement in understanding the kinetics and thermodynamics of isomerization events is hindered by experimental challenges such as the control of reaction selectivity and the characterization of reaction intermediates. Furthermore, the impact of solvent polarity and temperature on reaction speeds and product distributions adds complexity to the analysis of experimental data. Computational and spectroscopic tools are essential for understanding the mechanisms and routes of isomerization reactions. However, additional study is required to address the limits of the present methodologies.
Possible areas for future research and opportunities for further exploration:
In the future, the study of para- to meta-isomerization remains a promising area for further investigation and advancement. Progress in catalyst design, reaction optimization, and computational modeling has the potential to improve our comprehension of phenolic rearrangements and broaden the range of synthetic techniques accessible to chemists. Moreover, the utilization of para-to-meta-isomerization in new fields like materials science and green chemistry shows potential for tackling urgent societal issues and promoting sustainable solutions.
The prospects for study on para- to meta-isomerization are encouraging, offering possibilities for groundbreaking advancements and exploration in several domains of chemistry and materials science. Researchers will be able to develop novel methods for managing the selectivity and efficiency of isomerization reactions by making progress in catalyst design, reaction engineering, and computational modeling. Furthermore, including the conversion of para-to-meta-isomerization into sustainable manufacturing processes will aid in the advancement of environmentally friendly materials and technology. Scientists can tackle global concerns in energy, ecology, and health in the 21st century.
In conclusion:
To summarize, the transformation of phenols from para- to meta-isomer is a captivating event that has significant consequences in the field of chemistry and beyond. By clarifying the processes and practical uses of this basic process, scientists can open up new possibilities for chemical creation, drug discovery, and material design. This will lead to future advancements and discoveries in organic chemistry.
Distinct Frequently Asked Questions:
1). What are the primary factors that influence the process of para-to-meta-isomerization?
Temperature, solvent environment, and catalyst presence determine the efficiency and selectivity of isomerization reactions.
2). Is spontaneous para-to-meta-isomerization possible in phenolic compounds?
While certain phenols can spontaneously isomerize under certain conditions, external stimuli like heat or chemical catalysts frequently speed up or aid this process.
3). What are the practical uses of para-to-meta-isomerization in industry?
Para-to meta-isomerization is a commonly employed technique in the pharmaceutical and agrochemical sectors to alter the characteristics of organic compounds, such as their reactivity, stability, and biological activity.
4). What methods do researchers use to investigate the mechanisms of para- to meta-isomerization?
Scientists use a variety of experimental methods, including spectroscopy and kinetic analysis combined with computer modeling, to understand the mechanisms and reaction pathways involved in isomerization processes.
5). Are there any potential hazards or disadvantages linked to the process of para-to-meta-isomerization?
Although para- to para-meta-isomerization has advantages in molecular design and synthesis, it is important to carefully assess and address any potential environmental or safety issues that may arise from the use of specific catalysts or reaction conditions. Para-to-meta-isomerization.
For more chemistry blogs, visit chemistry Master