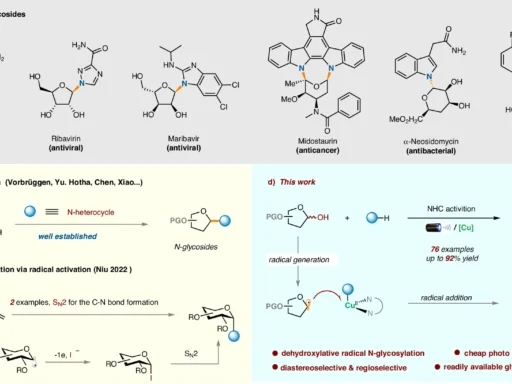
Table of Contents
Overview:
Noncovalent interactions are essential in chemistry and play a crucial role in a wide range of molecular processes, including the formation of biomolecule structures and the functioning of catalysts. Noncovalent interactions have attracted considerable attention in the field of catalysis because of their capacity to impact reaction routes, selectivity, and efficiency. An interesting instance of the interaction between noncovalent contacts and catalysis may be seen in the use of spirobipyridine ligands with iridium catalysts to enhance C-H activation processes. This article offers a comprehensive examination of this intriguing phenomenon, examining the fundamental concepts, empirical data, practical uses, and future possibilities.
Comprehending noncovalent interactions:
Noncovalent interactions are a type of chemical interaction that occurs between molecules or parts of molecules without the formation or breaking of chemical bonds. These interactions are often weaker than covalent bonds and include forces such as hydrogen bonding, van der Waals forces, and electrostatic forces.
Noncovalent interactions refer to a wide range of forces that bring molecules together without creating covalent bonds. The interactions involved are hydrogen bonding, π-π stacking, van der Waals forces, and hydrophobic interactions. Noncovalent interactions are very important in catalysis because they keep reactive intermediates stable, help substrates bind, and control how reactive catalysts are.
Significance in Catalysis:
Noncovalent interactions are important in catalytic processes because they have a significant impact on reaction kinetics and selectivity. These interactions stabilize transition states, enhance substrate recognition, and facilitate the release of products. Chemists can optimize catalysts by utilizing these interactions to improve their performance and selectivity for specific reactions. Therefore, it is crucial to comprehend the complexities of noncovalent interactions to create highly effective catalytic systems in many areas of synthetic chemistry.
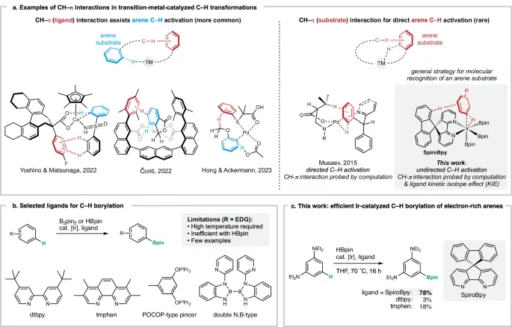
CH–π interactions in transition metal catalysis:
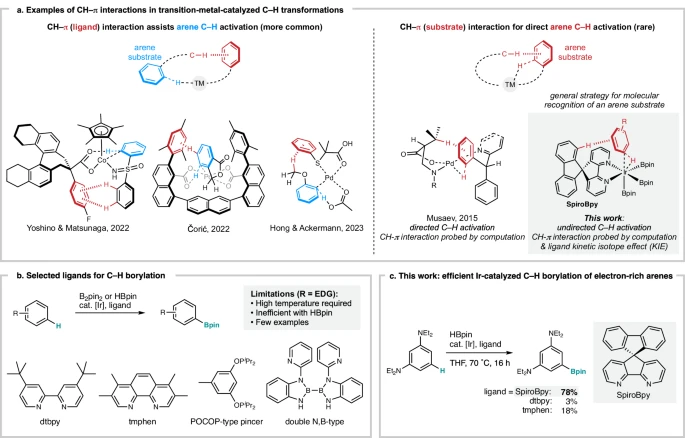
An Introduction to Spirobipyridine Ligands:
Structure and Properties:
Spirobipyridine ligands are a type of heterocyclic molecule that has a spirocyclic core consisting of two pyridine rings. The presence of the spirobipyridine motif gives these ligands distinctive structural stiffness and chelating capability, making them highly valuable in the field of coordination chemistry. The inflexible core structure of spirobipyridine ligands guarantees accurate arrangements of atoms around metal centers, therefore impacting the spatial configuration of catalytic processes. In addition, the nitrogen atoms in the pyridine rings make it possible for transition metals to bind, which helps make metal-ligand complexes that last.
Catalysis Applications:
Spirobipyridine ligands have structural characteristics that render them very suitable for a wide range of catalytic applications. Because of their ability to form complexes with metal ions and regulate the arrangement of atoms around them, ligands have become widely used in transition metal catalysis. Spirobipyridine-based compounds have been utilized in a wide array of processes, such as cross-coupling reactions, hydrogenation, and C-H activation. These ligands’ adjustable characteristics enable precise adjustment of catalytic properties, including activity, selectivity, and stability, making them highly valuable instruments in synthetic chemistry.
Challenges and Opportunities in Iridium-Catalyzed C–H Activation:
Significance of C-H Activation:
Carbon-hydrogen (C-H) activation is a highly effective approach for directly modifying organic compounds, allowing for the targeted incorporation of new functional groups into unreactive C-H bonds. This transformation eliminates the requirement for pre-functionalized substrates, simplifies the synthetic pathways, and reduces the production of trash. Iridium catalysts have proven to be highly efficient catalysts for C-H activation due to their capacity to perform in gentle circumstances and withstand a diverse array of functional groups.
Difficulties with C-H Activation:
Although there has been substantial advancement in the area of C-H activation, there are still some obstacles that remain. A primary factor is the preferential triggering of particular C-H bonds while other reactive sites coexist within a molecule. Furthermore, the pursuit of attaining elevated levels of catalytic efficiency and selectivity continues to be a primary objective for numerous C-H activation processes. To tackle these issues, it is necessary to create inventive catalyst design strategies that may overcome intrinsic substrate biases and reduce side reactions.
Scope of substrates:
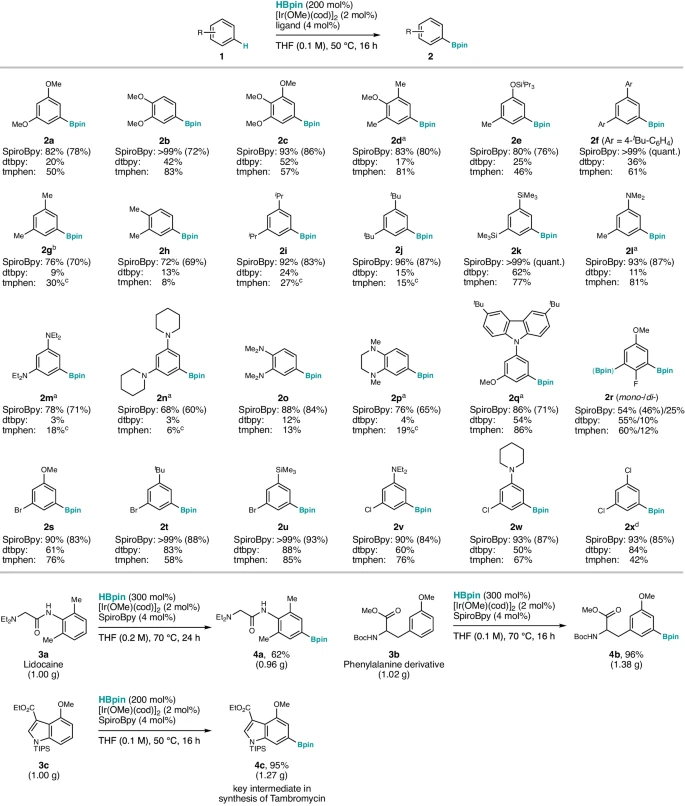
Noncovalent Interactions with Spirobipyridine Ligands: Mechanistic Insights:
Explanation and Importance:
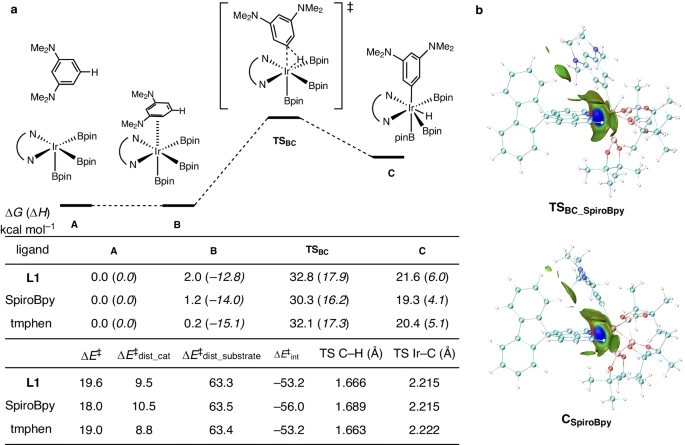
The integration of spirobipyridine ligands into iridium catalysts signifies a fundamental change in the realm of C-H activation. The non-covalent interaction between the ligand and metal center has a significant impact on the system’s catalytic activity and selectivity. The spirobipyridine ligand increases the iridium catalyst’s stability and aids in binding and activating the substrate by establishing a stable coordination complex. This interaction also affects the electrical characteristics of the metal core, hence impacting its reactivity towards C-H bonds.
Increased catalytic efficiency:
Experimental studies have shown convincing evidence that noncovalent interactions play a significant role in increasing the effectiveness of iridium-catalyzed C-H activation processes. Researchers studying kinetics have shown that catalysts with spirobipyridine ligands have faster reaction rates and more turnovers than regular catalysts. Furthermore, detailed scientific studies, including computational modeling and spectroscopic analysis, have provided valuable insights into the characteristics of noncovalent contact and how it affects the process of the reaction.
Mechanistic investigation:
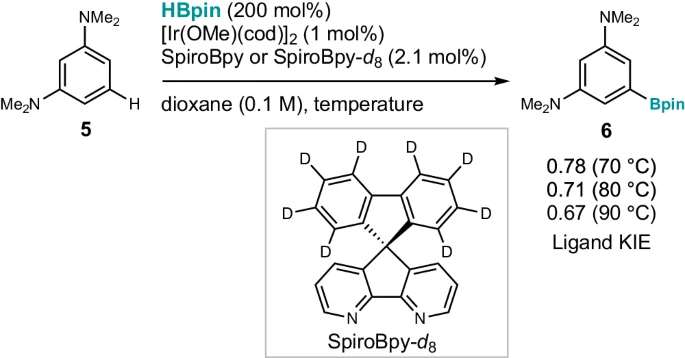
Experimental Evidence and Results: Insights from Studies:
Studies Supporting the Interaction:
Multiple empirical studies have confirmed the importance of noncovalent interactions in the process of iridium-catalyzed C-H activation. X-ray crystallography investigations have revealed the structural details of the catalytic complex, specifically the arrangement of atoms and the way they interact. These studies have shown the relationship between the spirobipyridine ligand and the iridium core. Moreover, we have utilized spectroscopic methods like NMR spectroscopy and UV-visible spectroscopy to elucidate the characteristics of the interaction and its influence on the stability and reactivity of the catalyst.
Comparative Analysis of Efficiency between Traditional Catalysts:
Comparative studies have shown that spiro bipyridine-based catalysts are more effective and selective than standard catalysts in terms of catalytic efficiency. For instance, in a benchmark C-H activation process, catalysts that included spirobipyridine ligands demonstrated superior turnover frequencies and reduced catalytic loadings in comparison to catalysts without ligands. The increased effectiveness of spiro bipyridine-based catalysts can be attributed to the stabilizing influence of the ligand on the catalytic species, resulting in accelerated reaction kinetics and an enhanced range of substrates. spiro bipyridine-based.
Applications in Organic Synthesis: Broadening the Range and Flexibility of Synthetic Methods:
Scope and Versatility:
Finding noncovalent interactions with spirobipyridine ligands has opened up new areas of organic synthesis and made it easier to make changes that work well and are specific. Spirobipyridine-based catalysts have a wide range of substrates they may act upon and exhibit great flexibility, making them highly valuable for synthesizing intricate compounds and constructing molecular frameworks. The catalysts have a wide range of applications in several fields of chemical research, including medicine and materials science.
Illustrations of Reactions Facilitated by the Interaction:
Organic synthesis has effectively used catalysts based on spirobipyridine in a range of synthetic reactions. Examples include the arylation, alkylation, and functionalization of different substrates under mild reaction conditions. The utilization of spirobipyridine ligands has notably facilitated the selective activation of C-H bonds within intricate molecules, resulting in the fast construction of molecular structures with exceptional efficiency and selectivity.
Computational investigations:
Future Directions and Potential Impacts:
Implications for Synthetic Chemistry:
Mapping the Path Forward Implications for the Field of Synthetic Chemistry
Finding noncovalent interactions with spirobipyridine ligands is a big step forward in catalysis that has many applications in synthetic chemistry. By utilizing the distinctive characteristics of these ligands, scientists can create catalysts that exhibit improved reactivity, selectivity, and sustainability. The capacity to specifically trigger C-H bonding in intricate molecules presents novel opportunities for the production of bioactive chemicals, natural products, and functional materials.
Potential areas for further investigation:
To fully maximize the potential of noncovalent interactions with spirobipyridine ligands in catalysis, several research pathways must be explored moving forward. These activities include the creation of new ligand frameworks with specific characteristics, investigations into reaction pathways’ mechanisms, and the use of these catalysts in asymmetric synthesis. Furthermore, it is crucial to make endeavors toward enhancing reaction conditions, broadening the range of substrates, and increasing the size of processes for industrial purposes to fully realize the potential of this technology.
Summary: A stimulus for creativity
To summarize, the noncovalent interaction between spirobipyridine ligands and iridium catalysts is a significant advancement in the area of C-H activation. Scientists have discovered novel opportunities for catalytic transformations by utilizing noncovalent interactions, resulting in more effective and environmentally friendly synthetic pathways. As we further understand the complexities of these interactions and their impact on catalysis, there is great potential for the creation of advanced catalysts in the future that possess exceptional reactivity and selectivity.
Frequently Asked Questions: Providing Answers to Your Urgent Inquiries
1). What is the mechanism by which noncovalent interactions with spirobipyridine ligands enhance catalytic efficiency?
The contact improves the catalytic species’ stability and promotes substrate binding and activation, resulting in faster reaction rates and higher yields.
2). Which specific C-H activation processes can be advantageous when employing this strategy?
Studies have shown that this interaction promotes a variety of transformations, including arylation, alkylation, and functionalization of olefins.
3). Are spirobipyridine ligands specific to iridium catalysts?
Researchers have also looked into the potential use of spirobipyridine ligands, created for iridium catalysts, with other transition metals in catalytic reactions.
4). Can different types of catalytic reactions utilize noncovalent interactions between ligands and other reactants?
Indeed, noncovalent interactions possess a wide range of practical uses in catalysis and can significantly improve the efficiency of catalysts in diverse reaction types that go beyond C-H activation.
5). What are the potential drawbacks or limitations of this approach?
Several problems, such as ligand synthesis, substrate compatibility, and scalability, necessitate additional exploration and optimization.
For more chemistry blogs, visit chemistry Master