Table of Contents
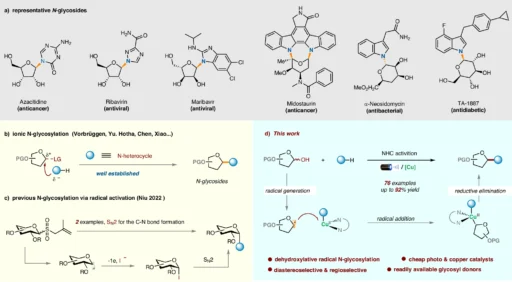
Within organic chemistry, the dehydroxylative radical N-glycosylation procedure is a notable development because it provides a new method for the efficient and accurate synthesis of intricate compounds. With the potential to completely transform the field of organic synthesis, this ground-breaking method made possible by copper metallophotoredox catalysis has attracted a lot of interest.
An Introduction to N-Glycosylation by Dehydroxylative Radicals:
The dehydroxylative radical N-glycosylation process changes heterocycles that use 1-hydroxy carbohydrates through radical intermediates. This creates glycosidic linkages in the end. This approach has clear benefits in terms of versatility and selectivity, setting it apart from conventional glycosylation tactics. Chemists and researchers looking for effective ways to synthesize complicated molecules are very interested in it as a result of its discovery. The importance of N-glycosides and the background of this research:
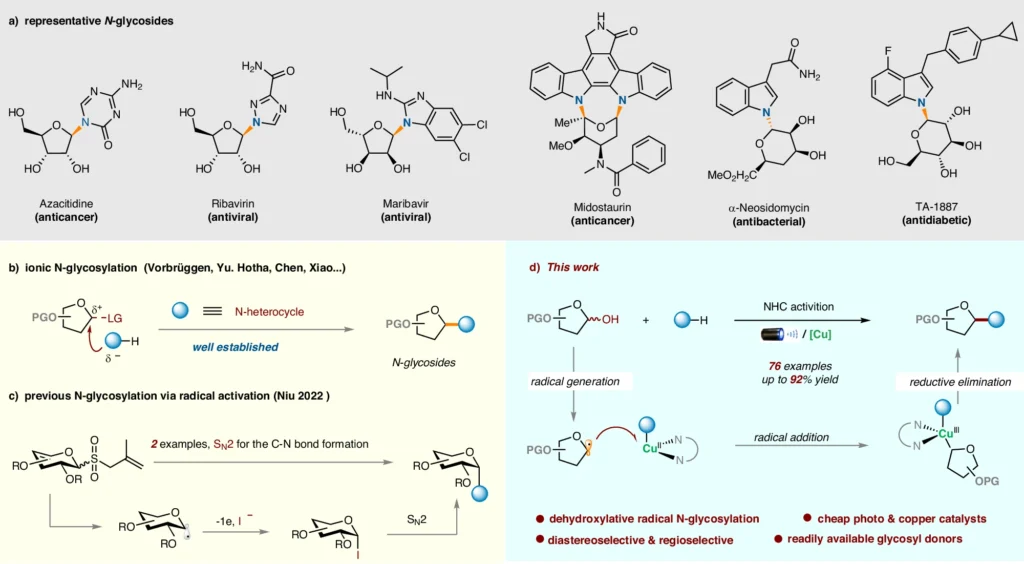
An Overview Of Heterocycles and 1-HydroxyCarbohydrates:
Heterocycles, with atoms other than carbon in their ring structure, are ubiquitous and essential elements of organic chemistry. The hydroxyl group at the C-1 position of 1-hydroxycarbohydrates, produced from carbohydrates, serves as a potential precursor for glycosylation reactions.
Catalysis by Copper Metallophotoredox:
Copper metallaphotoredox, which supports the production of radicals from heterocycles and 1-hydroxy carbohydrates when exposed to photoirradiation, mostly catalyzes the process of dehydroxylative radical N-glycosylation. Using the unique properties of copper complexes and light energy to make the necessary changes, this catalytic system creates bonds that last and work well.
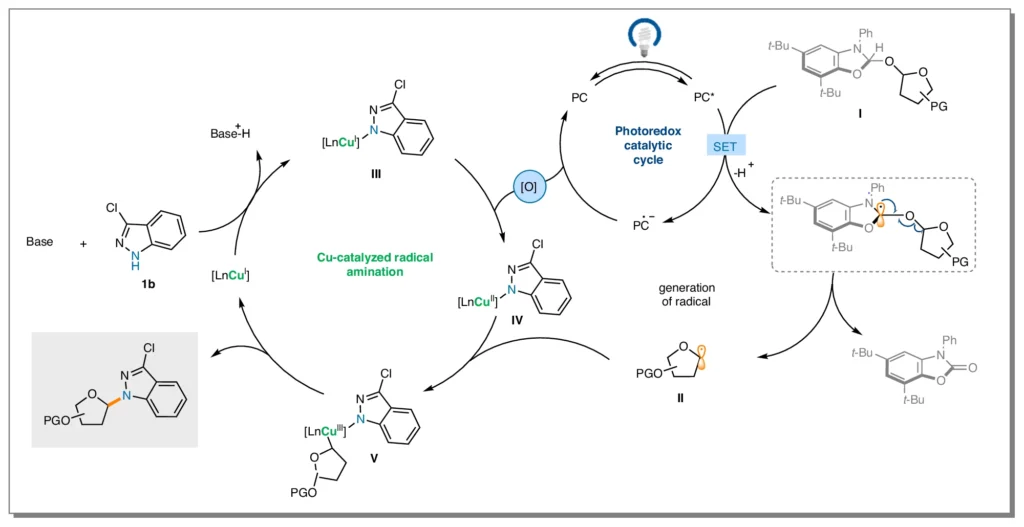
Important Ideas in the Reaction Mechanism:
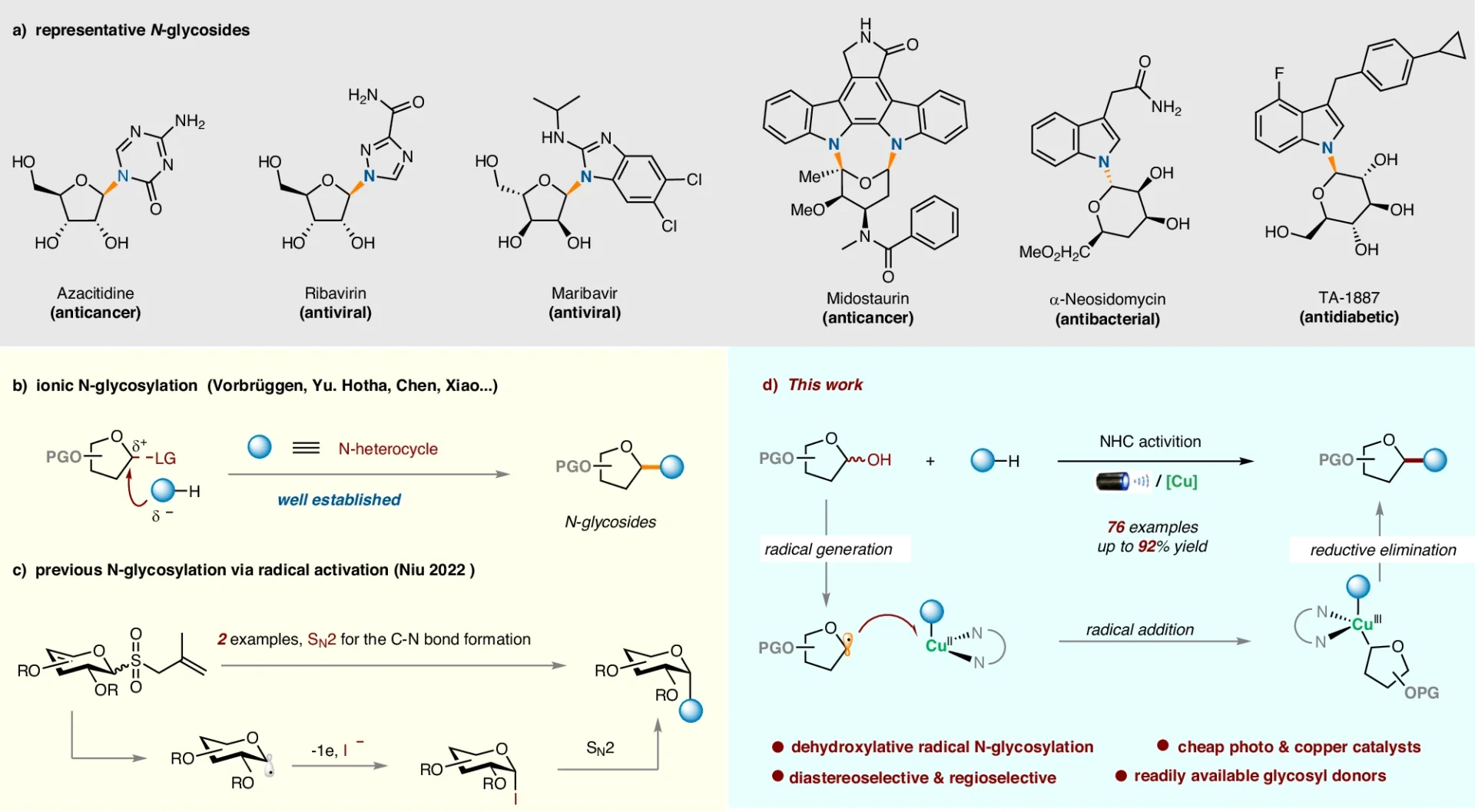
In the dehydroxylative radical N-glycosylation pathway, many important steps are taken, such as making radicals, activating functional groups, and making bonds. A key role that copper complexes play in mediating these processes is to remarkably faithfully orchestrate the complicated dance of electrons and facilitate the desired changes. Proposed Mechanism for the dehydroxylative radical N-glycosylation.
The substrate scope for N-heterocycles:
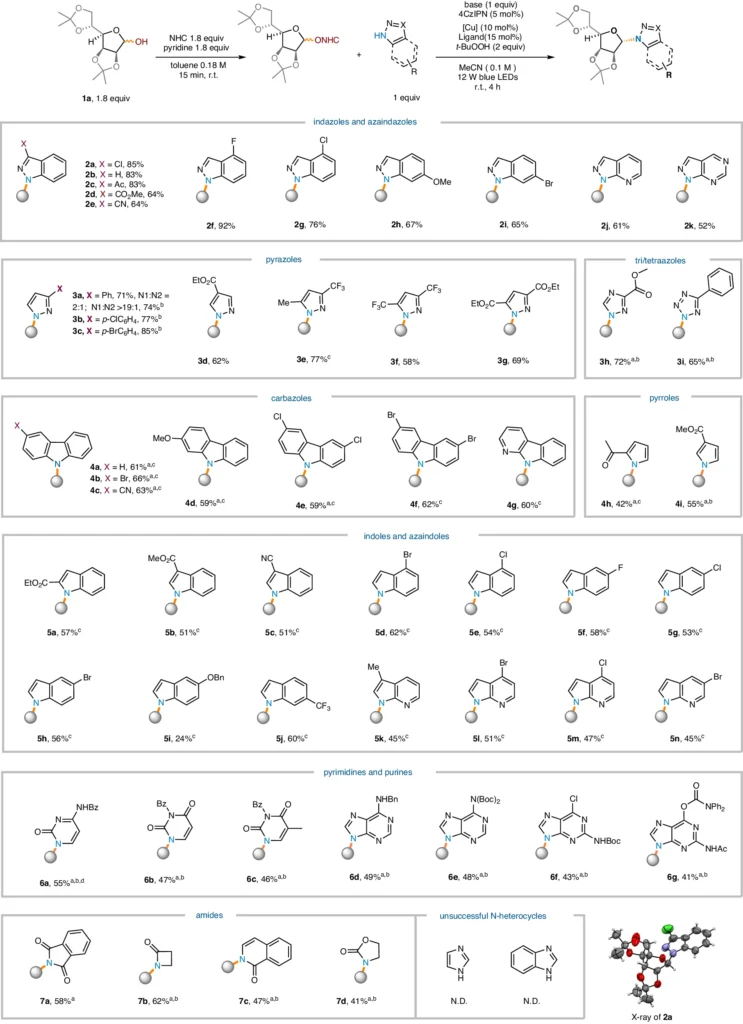
The reaction’s extent and bounds:
Dehydroxylative radical N-glycosylation has several applications, but its effectiveness is dependent on a few key parameters. Reaction circumstances, catalyst design, and substrate compatibility all have an impact on how a reaction turns out, emphasizing the importance of carefully examining and optimizing reaction parameters.
Dehydroxylative Radical N-Glycosylation Applications:
The synthesis of natural products, medicine, and materials science are just a few of the fields in which dehydroxylative radical N-glycosylation finds use. This technology opens up new directions in drug discovery, molecular engineering, and biomaterial design by facilitating the quick assembly of complex molecules.
New developments and research findings:
Researchers have discovered novel catalyst systems, reaction mechanisms, and substrate scopes in the field of dehydroxylative radical N-glycosylation, which has advanced significantly in recent years. These developments have increased the usefulness of this revolutionary methodology, opening the door for the creation of more effective and focused protocols.
Difficulties and Solutions:
Dehydroxylative radical N-glycosylation has difficulties, despite its potential. Obstacles to its broad implementation include side reactions, scalability, and substrate specificity. Nonetheless, scientists are aggressively tackling these obstacles to realize the full promise of this game-changing technology through creative catalyst design, reaction optimization, and mechanistic insights.
Analogous Study Using Conventional Glycosylation Techniques:
Glycosylation with dehydroxylative radicals is better than other glycosylation methods in several ways, including better reaction conditions, better selectivity, and a wider range of substrates. This technology simplifies the synthesis process and increases accessibility for researchers in a variety of domains by avoiding the requirement for preactivation and safeguarding group manipulations. Mechanistic Studies.
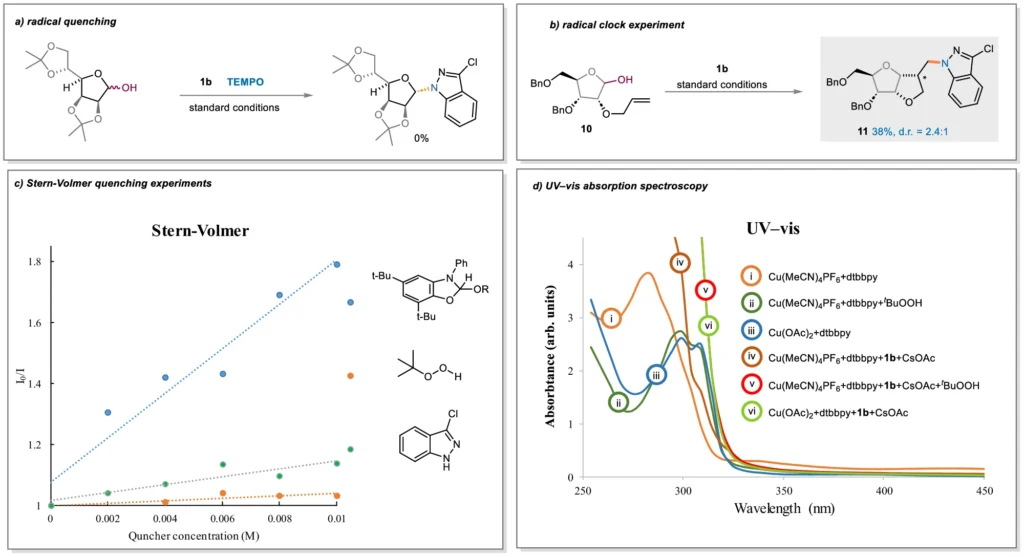
Industrial ramifications:
The production of fine chemicals, agrochemicals, and pharmaceuticals are just a few of the many possible industrial uses for dehydroxylative radical N-glycosylation. For enterprises looking for sustainable and affordable synthetic paths to valuable products, the capacity to quickly and precisely form complex glycosidic linkages has great significance.
Environmental and economic benefits:
Reduced waste creation, decreased energy usage, and fewer byproducts are just a few of the environmental benefits of dehydroxylative radical N-glycosylation. This methodology is in line with the concepts of green chemistry, which opens the door to more sustainable chemical synthesis techniques by reducing the use of harsh chemicals and optimizing reaction conditions.
Case Studies and Actual Instances:
Dehydroxylative radical N-glycosylation is a useful technique for synthesizing naturally occurring products, physiologically active chemicals, and functional materials. Several case studies and real-world examples demonstrate this. Examples of this novel methodology’s disruptive potential include the efficient synthesis of complex carbohydrates and the rapid creation of pharmaceutical intermediates.
Potential Futures and Upcoming Trends:
Continuous research aims to broaden its application, boost catalyst efficiency, and optimize catalyst design, indicating a promising future for dehydroxylative radical N-glycosylation. Emerging trends such as automation, computational modeling, and flow chemistry are likely to accelerate the development and adoption of this revolutionary technology.
Partnerships and Transdisciplinary Studies:
To advance the science of dehydroxylative radical N-glycosylation and open up new avenues for innovation, chemists, biologists, and materials scientists must work together. Researchers can take on challenging problems and provide ground-breaking, broadly applicable answers by utilizing a variety of interdisciplinary methodologies and specialized knowledge.
In conclusion:
In conclusion, dehydroxylative radical N-glycosylation is a big change in the field of organic synthesis because it makes it possible to make strong and useful glycosidic bonds. With the help of copper metallaphotoredox catalysis, this ground-breaking method has the potential to completely change chemical manufacturing, material research, and drug development. We anticipate a dramatic increase in the influence of dehydroxylative radical N-glycosylation on science and business with the development of new applications and research.
Particular FAQs:
1). Why is N-glycosylation by dehydroxylative radicals special?
Dehydroxylative radical N-glycosylation, which uses copper metallaphotoredox catalysis and radical intermediates, provides an alternative to conventional glycosylation techniques by facilitating the rapid and selective synthesis of bonds.
2). What role does the reaction’s copper metallophotoredox catalysis play?
When exposed to light, copper metallaphotoredox catalysis makes the changes that need to happen very quickly and efficiently by making it easier for radicals to form from heterocycles and 1-hydroxycarbohydrates.
3). Which dehydroxylative radical N-glycosylation applications are the most important ones?
Dehydroxylative radical N-glycosylation is a fast and effective method for synthesizing complex molecules and finds use in the synthesis of natural products, medicines, materials research, and other fields.
4). What are the main difficulties this methodology presents?
Some of the difficulties include scalability, side reactions, and substrate specificity. However, ongoing research endeavors focus on using catalyst design and reaction optimization to address these issues.
5). What is the role of dehydroxylative radical N-glycosylation in sustainability?
Dehydroxylative radical N-glycosylation supports green chemistry by providing more environmentally friendly alternatives to conventional synthesis techniques by limiting waste production, using less energy, and optimizing synthetic pathways.
6). What long-term effects might the N-glycosylation of dehydroxylative radicals have on business and research?
The widespread use of dehydroxylative radical N-glycosylation provides effective and sustainable synthetic pathways to valuable compounds, which could transform the production of fine chemicals, agrochemicals, and pharmaceuticals.
7). How can scholars collaborate to advance the field of N-glycosylation of dehydroxylative radicals?
Researchers from a variety of fields, such as chemistry, biology, and materials science, must work together to explore new applications, improve reaction conditions, and overcome significant obstacles in the field.
8). Does dehydroxylative radical N-glycosylation have any possible hazards or disadvantages?
While dehydroxylative radical N-glycosylation offers numerous benefits like efficiency and selectivity, one must also consider the associated hazards. These include side reactions and the formation of byproducts. Careful planning and reaction condition optimization can help reduce these risks.
9). How does innovation help the field of N-glycosylation of dehydroxylative radicals advance?
Whether through the creation of novel catalyst systems, the investigation of new reaction processes, or the use of emerging technologies, innovation is essential for propelling advancement in the field of dehydroxylative radical N-glycosylation. Researchers can open up new avenues for innovation and discovery in organic synthesis by pushing the limits of current knowledge and technology.
For more chemistry blogs, visit chemistry Master