Table of Contents
An Overview of Desaturative Catalysis:
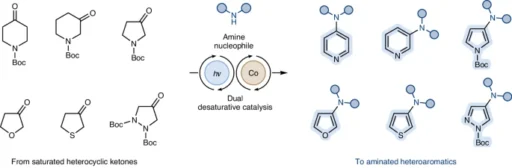
Desaturative catalysis is a leading technique in contemporary organic synthesis, providing a revolutionary method for modifying heteroaromatic molecules. Desaturative catalysis utilizes the reactivity of carbon-carbon double bonds to directly add nitrogen-containing functional groups to aromatic rings. This article examines the overall technique for converting both electron-rich and electron-poor heteroaromatic compounds via desaturative catalysis.
Comprehending Heteroaromatic Compounds:
Heteroaromatic compounds are a fundamental part of contemporary organic chemistry. They are defined by the presence of at least one heteroatom, such as nitrogen, oxygen, or sulfur, within the aromatic ring system. Their varied electrical properties make them essential in the production of medicines, agrochemicals, and innovative materials.
Challenges and Strategies in Electron-Rich Heteroaromatics:
Desaturative catalysis is a potential approach for the amination of electron-rich heteroaromatics; however, it is not without its difficulties and complexities.
Mechanism of Desaturation:
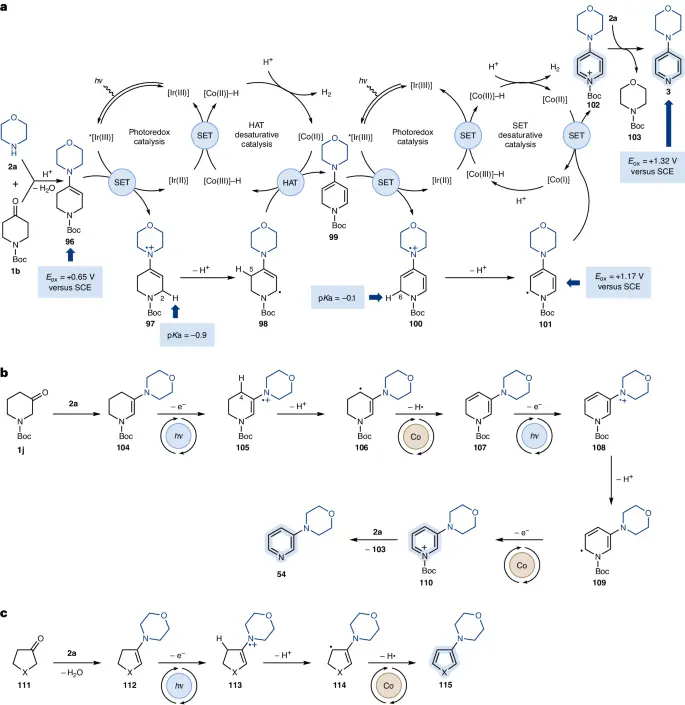
The process of reducing the saturation of heteroaromatic compounds that contain an excess of electrons is usually achieved by activating a carbon-hydrogen bond next to the heteroatom. Coordination of the catalyst to the heteroaromatic substrate facilitates this crucial step. Then, the C-H bond undergoes oxidative addition.
Selection of a Catalyst:
The careful choice of catalysts is crucial for the success of desaturative catalysis since they determine the specific location and effectiveness of the amination reaction. Transition metal complexes, such as palladium and ruthenium catalysts, have become well-established for facilitating desaturative reactions.
Range of Substrates:
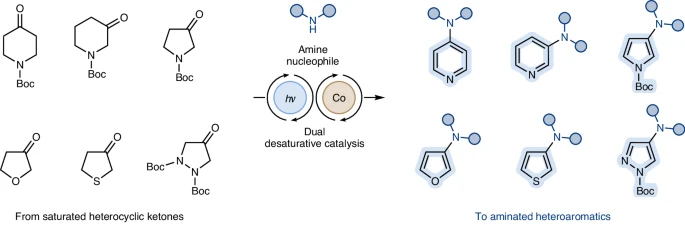
It is crucial to thoroughly investigate the range of substrates that can be used in desaturative amination processes to fully utilize their capabilities. Although heteroaromatics that have an excess of electrons usually show increased reactivity, there are ongoing efforts to create catalyst systems that can activate substrates with a deficiency of electrons. This would expand the range of synthetic methods available.
Addressing Reactivity Challenges in Electron-Deficient Heteroaromatic Compounds:
Activating heteroaromatic compounds that don’t have enough electrons is very hard and needs creative solutions to get around their natural limits on reactivity.
Impact of Electron Density:
Adding substituents that donate electrons or using catalysts with an excess of electrons can enhance the reactivity of heteroaromatic compounds with a deficiency of electrons. These interventions enhance the activation of C-H bonds next to the heteroatom, making amination processes more efficient.
Functional Group Tolerance:
Functional Group The ability of a chemical reaction or process to accommodate or accept different functional groups without hindrance or effect is known as tolerance.
Electron-withdrawing functional groups often hinder the amination of heteroaromatics using desaturative catalysis. Developing catalysts that have improved tolerance for these functional groups is a crucial way to broaden the range of synthetic reactions.
Recent Progress in Amination Methods:
Over the past ten years, there have been significant advancements in catalytic amination, thanks to improvements in ligand design, reaction engineering, and understanding of the underlying mechanisms.
Designing ligands for selectivity:
Custom-designed ligands play a crucial role in providing high selectivity for desaturative amination processes. When ligands are used, they can selectively activate C-H bonds in certain locations. This makes it possible for complex nitrogen-containing heterocycles to form very quickly.
Optimization of reaction conditions:
Optimizing reaction parameters, such as temperature, solvent choice, and catalyst amount, has a big effect on how well and specifically desaturative catalysis works. The development of gentle and sustainable methods for reactions with a wide range of heteroaromatic compounds is an ongoing effort.
Utilizations of Desaturative Catalysis:
The wide range of applications for desaturative catalysis is seen in various fields, including medicine and materials research.
Pharmaceutical sector:
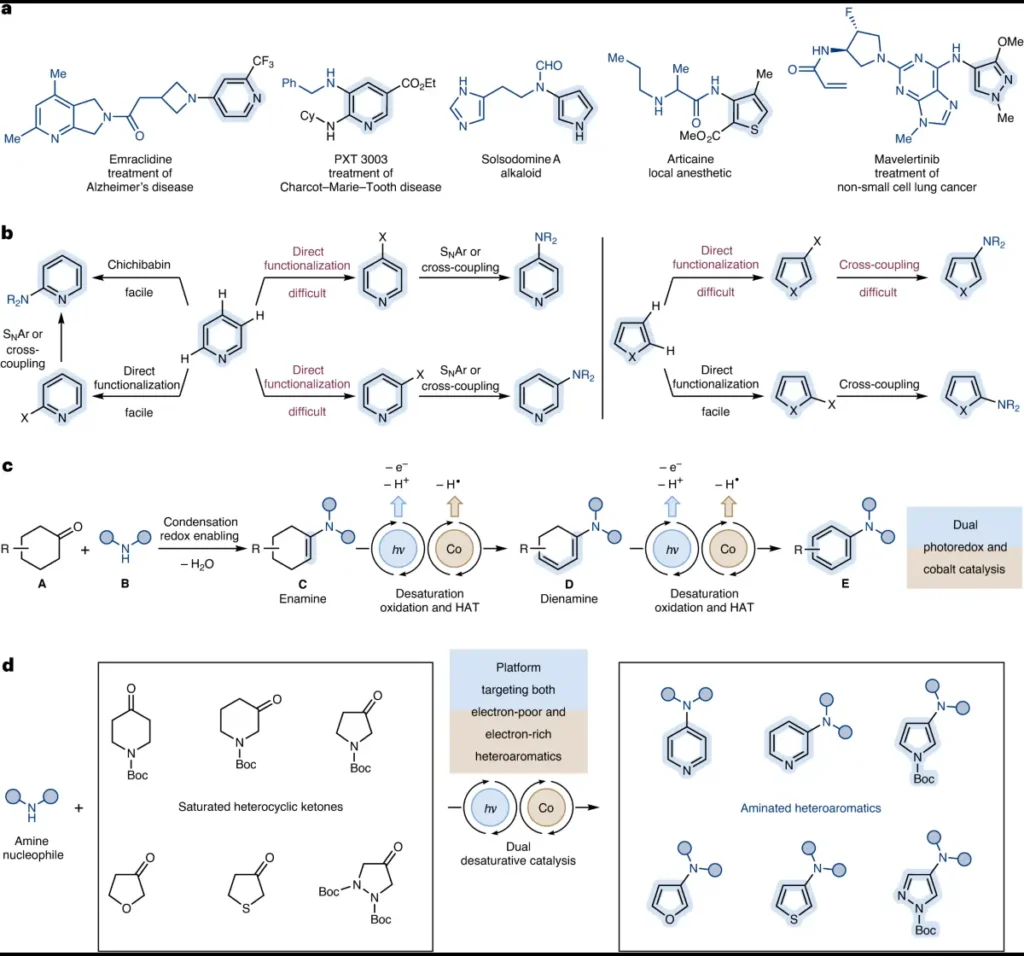
Desaturative catalysis is a quick and easy way to make a lot of different nitrogen-containing heterocycles, which is important for quickly making possible drugs and molecular probes. This accelerated access enables rapid investigation of structure-activity connections, thereby expediting the drug discovery process.
The study focuses on the properties, structure, and performance of materials:
Heteroaromatic compounds play a crucial role in the creation and production of useful materials, such as organic semiconductors, dyes, and catalysts. Desaturative catalysis allows the easy creation of customized heteroaromatic frameworks, enabling the deliberate development of materials with specific functions.
Obstacles and Prospects for the Future:
Despite its progress, desaturative catalysis still faces several obstacles on its path to wider adoption and use.
Scalability:
Scalability refers to the ability of a system or process to handle an increasing amount of work or data without compromising its performance or efficiency.
Converting small-scale desaturative amination reactions in the laboratory into large-scale industrial processes presents significant difficulties, including maintaining catalyst stability, improving reaction efficiency, and managing waste. To bridge this gap in translation, it is crucial to build scalable and sustainable synthetic techniques.
Factors to be taken into account in the field of green chemistry:
The need to adopt green chemistry concepts is significant in the development of desaturative catalysis. The efforts to create environmentally friendly catalytic systems and reaction protocols demonstrate a dedication to reducing the environmental impact of chemical synthesis.
In conclusion:
To summarize, desaturative catalysis demonstrates modern synthetic chemistry’s cleverness by providing a flexible and efficient method for creating nitrogen-containing heterocycles. This innovative method has caused significant changes in various fields, such as pharmaceuticals and materials research, by utilizing the reactivity of carbon-carbon double bonds.
Distinct Frequently Asked Questions:
1). What are the differences between desaturative catalysis and standard amination methods?
Desaturative catalysis gets rid of the need for pre-functionalized substrates or stoichiometric reagents, which makes it easier to make heterocycles that contain nitrogen.
2). What are the main elements that affect the selectivity of desaturative amination reactions?
The catalyst selection, substrate structure, and reaction conditions all have a significant impact on the specific location and type of chemical bonds that are modified during desaturative catalysis.
3). Is it possible to apply desaturative catalysis to heterocyclic frameworks other than aromatics?
Although the primary focus has been on aromatic heterocycles, there are ongoing efforts to expand desaturative catalysis to include aliphatic and fused-ring heterocyclic systems.
4). What factors impede the ability of desaturative amination processes to scale up?
Scalability issues include catalyst stability, reaction efficiency, and waste management. These challenges require collaborative efforts to design methods that are practical for industrial use.
5). What are how researchers might contribute to the advancement of desaturative catalysis?
Further investigation into catalyst design, optimization of reactions, and incorporation of green chemistry concepts will facilitate the development of more effective and environmentally friendly synthetic methods.
For more chemistry blogs, visit chemistry Master