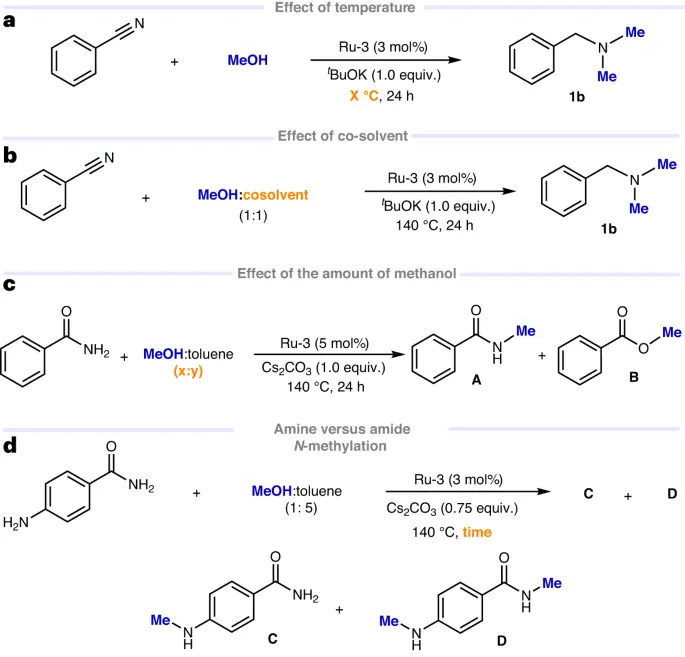
Table of Contents
Methanol, commonly known as wood alcohol because of its historical derivation from wood, is a basic C1 precursor in chemical synthesis. This substance’s versatility and significance stem from its ability to act as a precursor for a wide range of useful compounds in multiple industries. This thorough investigation examines the characteristics of methanol, its various methods of manufacture, its uses in chemical synthesis, its impact on the environment, the obstacles to its widespread use, and the potential it has for sustainable practices and technological advancements.
Properties of Methanol:
Physical properties:
It is a transparent, easily evaporating liquid with a characteristic scent. The boiling point of this substance is 64.7°C, and its density is roughly 0.79 g/mL. It has a high degree of miscibility with water, ethanol, ether, and other organic solvents.
Chemical properties:
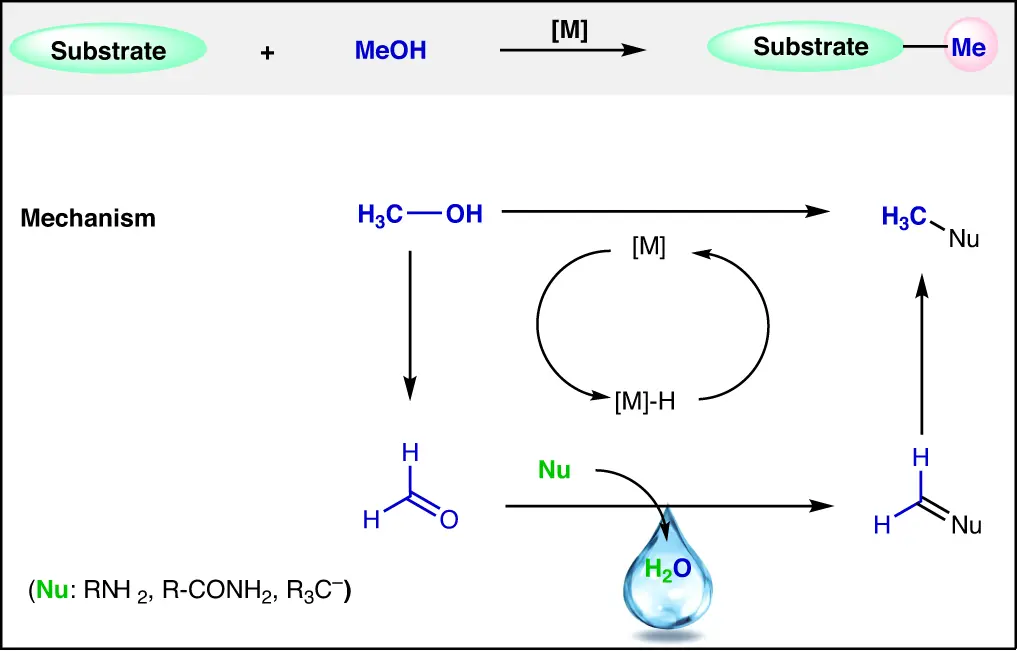
Properties that relate to the behavior and reactions of substances at the molecular level. It is the most basic alcohol, composed of a methyl group (-CH₃) connected to a hydroxyl group (-OH). This chemical structure in methanol gives it polarity, allowing it to effectively dissolve both polar and nonpolar compounds. It undergoes a range of chemical processes, such as oxidation to make formaldehyde (HCHO) and combustion to produce carbon dioxide (CO2) and water (H2O).
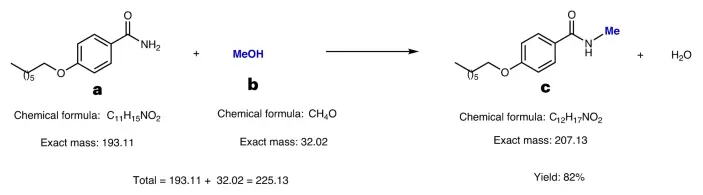
Mechanism:
Methods for Producing CH3OH:
Sources derived from nature:
Historically, the method of destructive distillation of wood produced CH3OH, leading to its alternate moniker, “wood alcohol.” Currently, gasification and the subsequent creation of syngas (a mixture of carbon monoxide and hydrogen) can still use biomass for methanol synthesis.
Methods of synthesis:
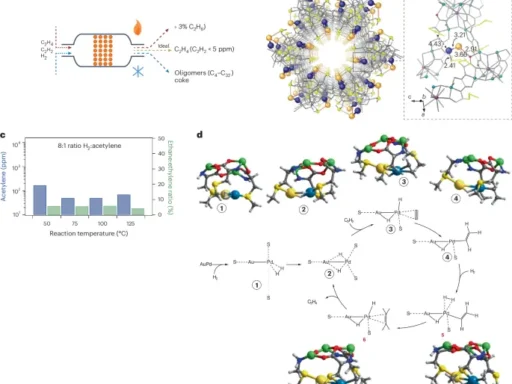
The catalytic conversion of synthesis gas (syngas), a mixture of carbon monoxide (CO) and hydrogen (H2), is the primary method for producing synthetic CH3OH. The procedural gas serves as the primary raw material for the process, also known as steam reforming or methane steam reforming. production from syngas is commonly carried out using a copper-based catalyst under high temperatures and pressures.
Utilization of CH3OH in Chemical Synthesis:
It is an important raw material in the production of many chemicals and materials. It acts as a starting point for the manufacturing of formaldehyde (HCHO), acetic acid (CHCOOH), methyl tert-butyl ether (MTBE), dimethyl ether (DME), and numerous hydrocarbons. Two prominent procedures that employ methanol as a primary substance are methanol-to-olefins (MTO) and methanol-to-hydrocarbons (MTH). Methanol-to-olefins (MTO) is a process that transforms methanol into ethylene (C₂H₄) and propylene (C₃H₆), which are light olefins. On the other hand, methanol-to-hydrocarbons (MTH) is a process that converts methanol into hydrocarbons that fall within the gasoline range.
CH3OH as a Sustainable Resource:
Evaluating its Environmental Footprint:
Utilizing methanol as a C1 building block aligns with sustainability objectives, as it can originate from renewable sources such as biomass and municipal solid waste (MSW). Its combustion exhibits reduced emissions of greenhouse gases in comparison to conventional fossil fuels, rendering it a propitious constituent in the shift towards more environmentally friendly energy sources.
Methods that are long-lasting and environmentally friendly:
Efforts to advance sustainable methanol production include the development of carbon capture and utilization (CCU) technology, which seeks to reduce CO2 emissions linked to methanol synthesis. Furthermore, ongoing research aims to enhance the integration of renewable energy sources like solar and wind power into methanol manufacturing methods. This involves exploiting these sources to produce hydrogen and, subsequently, generate syngas.
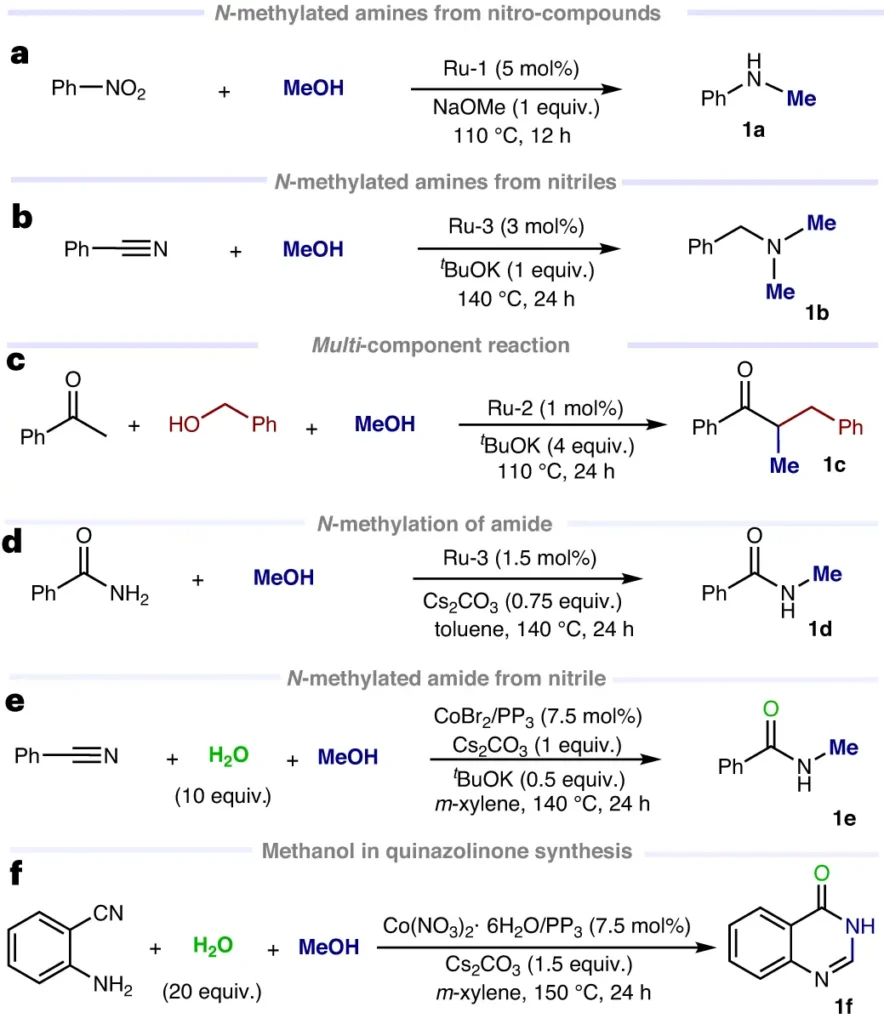
Obstacles and Prospects for the Future:
Various obstacles hinder the widespread use of it, despite its high versatility and numerous environmental advantages. These factors include the need for ongoing scientific progress to improve process efficiency, the ability to compete with traditional petrochemical methods in terms of cost, and the requirement to adhere to regulations concerning safety, health, and environmental effects. Nevertheless, the prospects for methanol as a renewable and sustainable C1 building block are still positive. Currently, ongoing research and development endeavors focus on tackling these obstacles and broadening their utilization in various industries.
In conclusion:
To summarize, It plays a crucial role as a fundamental component in contemporary chemical synthesis, offering a flexible and environmentally friendly substitute for traditional raw materials. Due to the increasing focus on sustainability and technical advancement, It is becoming increasingly important in the shift towards more environmentally friendly and efficient chemical processes.
Frequently Asked Questions:
1). Is CH3OH suitable for chemical synthesis?
Chemical synthesis frequently uses methanol, but its toxicity and flammability require careful handling. It is important to take appropriate safety precautions, such as ensuring there is enough ventilation and wearing personal protection equipment (PPE) when storing, handling, and disposing of it.
2). What are the differences between methanol and ethanol?
Methanol (CH₃OH) and ethanol (C₂H₅OH) are two distinct types of alcohols that vary in terms of their chemical composition and characteristics. Methanol is very poisonous and can result in vision loss or fatality if consumed, whereas ethanol, the form of alcohol included in alcoholic drinks, is normally less harmful to humans.
3). Is CH3OH a viable fuel option?
In CH3OH fuel cells, direct fuel cells (DMFCs), and as a mixture with gasoline in flex-fuel cars, methanol serves as a viable fuel substitute. Its use as a fuel for transportation presents promising advantages in terms of emissions reduction and reliance on fossil fuels.
4). What are the environmental benefits of using CH3OH?
Renewable methanol plays a crucial role in mitigating carbon emissions and advancing sustainable practices within the chemical industry. This material’s adaptability as a raw material for various chemical processes aligns with the growing demand for environmentally friendly alternatives to conventional petrochemicals.
5). What is the projected future for CH3OH production and utilization?
Progress in renewable energy technology, carbon capture and utilization strategies, and pioneering chemical processes intricately link to the future of its production. Ongoing research and investment in sustainable methanol production will be crucial in influencing the chemical industry’s shift towards a more environmentally friendly and sustainable future.
For more chemistry blogs, visit chemistry Master