Table of Contents
The convergence of adsorption and bacterial activity within environmental research offers fascinating opportunities for addressing water contamination. Nanoparticles, specifically Ag nanoparticles modified with Nd2O3, have become useful tools in this field because they have better adsorption properties and help break down harmful contaminants like methylene blue.
Introduction to Adsorption and Bacterial Performance:
Adsorption refers to the buildup of pollutants onto a surface. This mechanism is critical in wastewater treatment because it effectively removes contaminants from water matrices. Furthermore, bacteria’s efficacy is essential to the biodegradation process, in which microorganisms decompose organic contaminants into less toxic compounds. The combination of adsorption and bacterial activity shows significant potential for tackling pollution concerns.
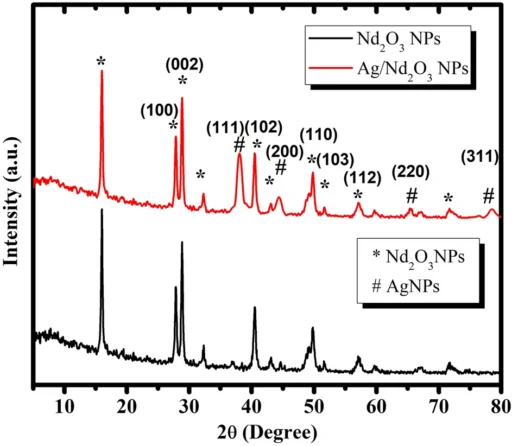
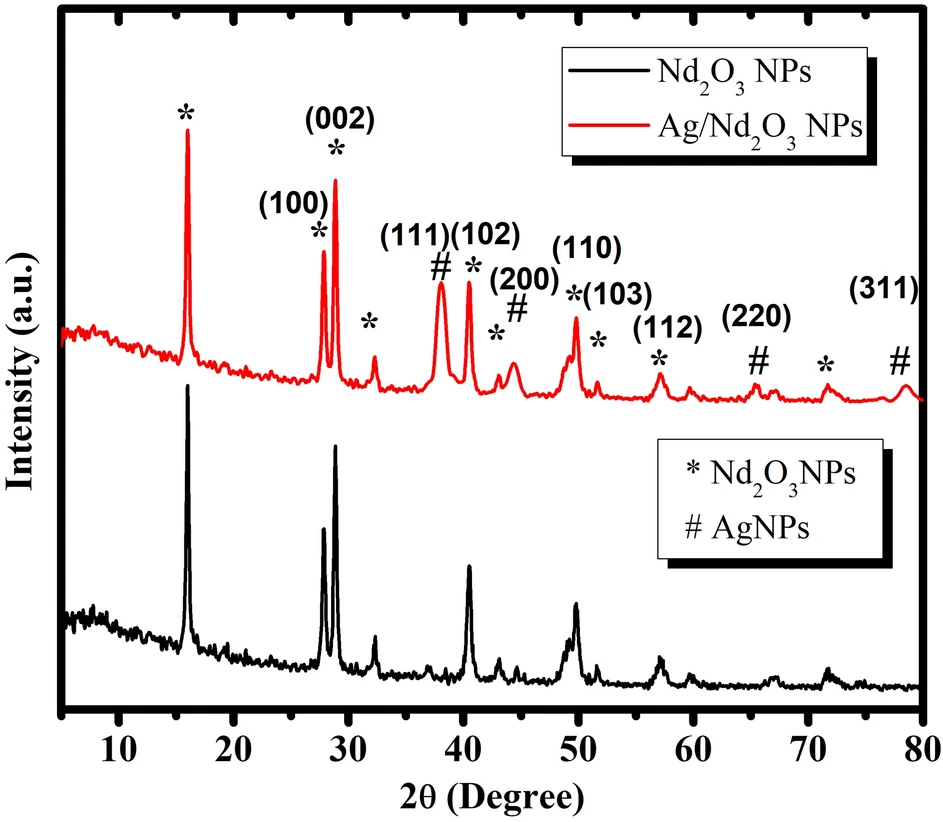
Nanoparticles possess distinctive adsorption capabilities as a result of their diminutive size and extensive surface area. When suitably altered, nanoparticles can specifically trap contaminants from water-based solutions. Nd2O3-modified Ag nanoparticles are a unique type of man-made nanomaterials that have better adsorption properties, which makes them very useful in environmental applications. XRD of Pure Nd2O3 NPs, and Ag/Nd2O3NPs.
An Exploration of Nanoparticles and Their Alterations:
Nanoparticles are particles with extremely small sizes, typically ranging from 1 to 100 nanometers in diameter. Their diminished size confers unique physicochemical characteristics, such as enhanced surface area and reactivity. We subject nanoparticles to various changes, such as surface functionalization and doping with different elements, to enhance their effectiveness in environmental remediation. The modifications customize nanoparticles for specific types of pollutants and improve their interactions in water-based settings.
Nd2O3-modified Ag nanoparticles are a type of silver nanoparticle that has been further modified using neodymium oxide (Nd2O3). This change improves the stability, reactivity, and adsorption capability of the materials, making them highly efficient at absorbing and breaking down pollutants.
The role of Nd2O3-modified Ag nanoparticles in adsorption is discussed:
Various characteristics, such as their surface area, surface chemistry, and porosity, determine the ability of nanoparticles to adsorb substances. The Nd2O3-modified Ag nanoparticles have a lot of surface area compared to their volume. This means that they can hold more pollutants per unit mass than other adsorbents. Furthermore, we can customize the surface chemistry of these materials to selectively attract specific contaminants through electrostatic interactions, hydrogen bonding, or chemical complexation.
When mixed with water that contains methylene blue, Ag nanoparticles that have been treated with Nd2O3 form stable complexes with the dye molecules that remove them effectively from the solution. Depending on the prevailing environmental conditions, the process of adsorption can occur quickly and reverse itself.
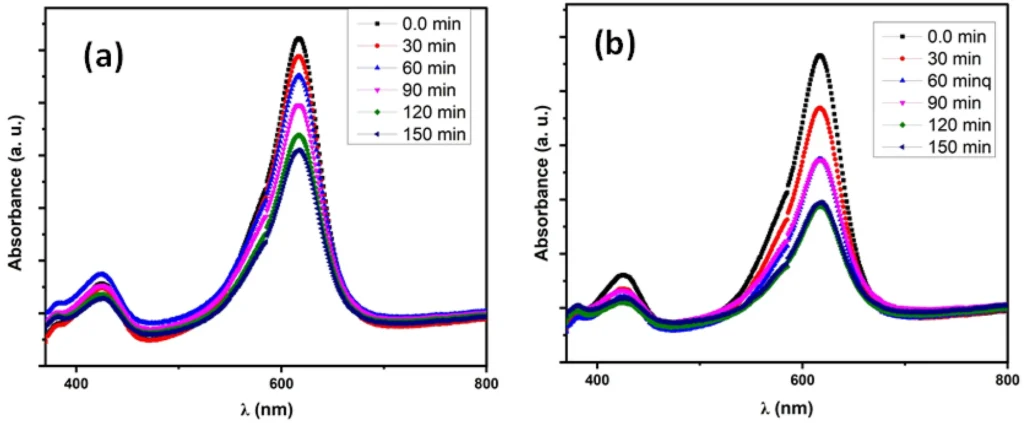
The degradation of MB using (a) pristine Nd2O3 NPs, (b) Ag/Nd2O3NPs.
Accelerated Breakdown of Methylene Blue:
Many industries, including textiles, printing, and leather processing, frequently use methylene blue, a man-made coloring agent. Nevertheless, the release of this substance into water systems presents environmental hazards because of its long-lasting nature and potential harm to aquatic creatures. Adding neodymium oxide Nd2O3-modified Ag nanoparticles speeds up the breakdown of methylene blue by using both photocatalytic and catalytic processes.
Sometimes, like when they are exposed to light or certain reactive substances, Ag nanoparticles that have been treated with Nd2O3 make free radicals or activate oxygen species that break down methylene blue molecules. The degradation route converts methylene blue into less complex and less hazardous substances, minimizing its environmental consequences.
Experimental configuration and methodology:
We use comprehensive experimental techniques to evaluate the adsorption and degradation abilities of Ag nanoparticles enhanced with Nd2O3. Through batch experiments, nanoparticles are mixed with set amounts of contaminants, and then changes in the levels of contaminants are watched as time goes on. Performance optimization involves methodically varying parameters such as pH, temperature, and nanoparticle dosage.
We employ UV-Vis spectroscopy, Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) to analyze the interactions between nanoparticles and pollutants and verify the degradation products. Biological assays can assess microbial communities and determine the effects of nanoparticle-treated liquids.
Outcomes and discoveries:
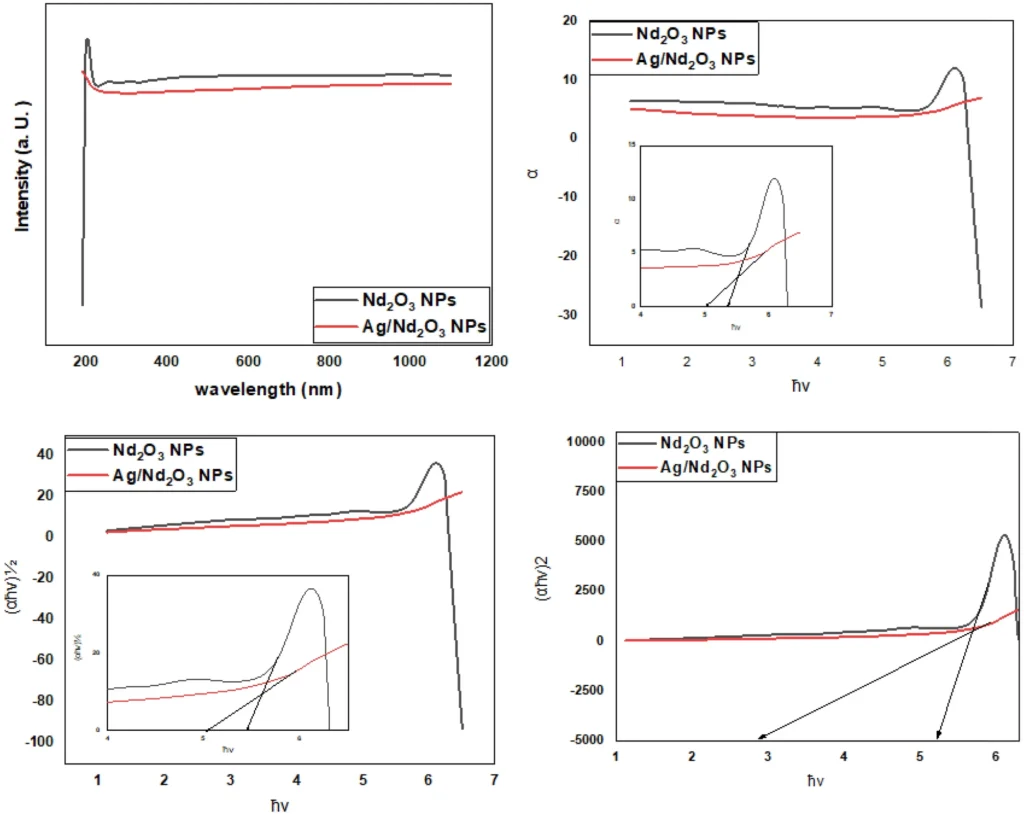
According to the test results, Ag nanoparticles that have been treated with Nd2O3-modified Ag are better at absorbing things than regular adsorbents. The nanoparticles efficiently eliminate methylene blue from water-based solutions, attaining excellent removal rates even at low levels of concentration. Moreover, the presence of these nanoparticles enhances the degradation kinetics of methylene blue, emphasizing their catalytic activity.
The analysis of nanoparticle surfaces identifies distinct binding sites responsible for pollutant adsorption. Spectroscopic examination confirms the chemical changes in methylene blue molecules upon exposure to nanoparticles, illuminating the pathways of degradation.
Significance for Environmental Applications:
The use of Nd2O3-modified Ag nanoparticles has important implications for environmental cleanup. Their effective ability to remove pollutants reduces the need for traditional treatment procedures, thereby lowering the use of chemicals and energy consumption. Furthermore, the catalytic breakdown of contaminants provides a sustainable method for purifying water, contributing to worldwide efforts to create cleaner ecosystems.
Nanoparticle-based techniques provide distinct advantages over standard treatment technologies, particularly in terms of their effectiveness and cost efficiency. Large-scale wastewater treatment systems highly suit nanoparticles due to their easy scaling up in both synthesis and application. Antibacterial behavior of Pure Nd2O3 NPs, and Ag/Nd2O3NPs.
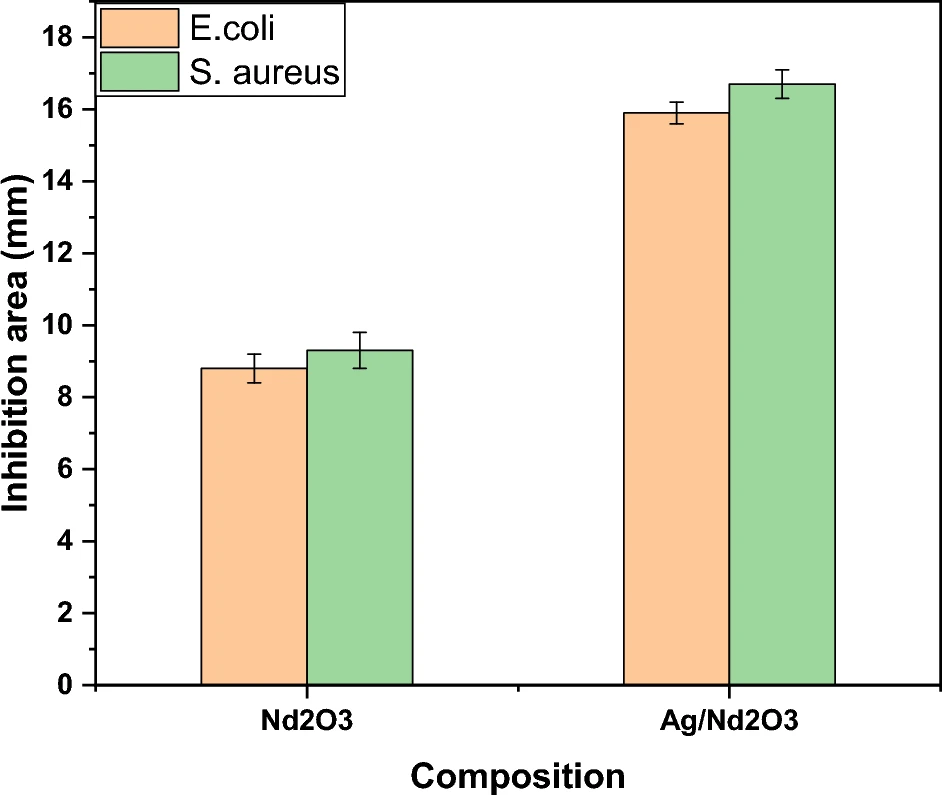
Potential areas for further exploration and opportunities for further research exist:
Despite the significant advancements in nanoparticle-based cleanup, numerous areas still require further investigation in the future. It is crucial to optimize the processes used to synthesize nanoparticles to improve their stability and reactivity, which is necessary for their commercial use. Looking into how nanoparticles and microbial communities affect each other could lead to new biotechnological ways to deal with harmful combinations of pollutants.
Furthermore, it is critical to prioritize the resolution of concerns related to the release of nanoparticles and their influence on the environment to achieve universal acceptance and use. Life cycle assessments and risk studies will guide the safe advancement and implementation of nanoparticle technologies in various environmental contexts.
In conclusion:
Overall, the utilization of Nd2O3-modified Ag nanoparticles shows great potential for progress in the realm of environmental nanotechnology. Their ability to both adsorb and catalyze makes them promising for tackling water pollution issues, especially in the area of dye removal. Ongoing research and innovation will continue to enhance the potential of these nanoparticles, facilitating the development of sustainable solutions for environmental cleanup.
Frequently Asked Questions:
1). What is the difference in pollutant removal efficiency between Nd2O3-modified Ag nanoparticles and traditional adsorbents?
Nanoparticles of Nd2O3-modified Ag have better adsorption properties and can be tailored to specific contaminants, making them more useful and flexible than regular adsorbents.
2). Which factors influence the rate at which Ag nanoparticles treated with Nd2O3 break down methylene blue?
Factors such as the amount of nanoparticles used, the intensity of light exposure, and the pH level of the solution play an important role in determining the speed and degree of methylene blue degradation.
3). Is there any risk associated with nanoparticle-based remediation?
A thorough examination and continuous investigation are required to fully understand the ecological consequences and long-term effects of nanoparticle emissions on ecosystems.
4). Is it possible to recycle or reuse Nd2O3-modified Ag nanoparticles in wastewater treatment?
Researchers are currently working to develop methods for regenerating and reusing nanoparticles to cut waste and improve resource exploitation efficiency.
5). What is the level of scalability for the manufacture of Nd2O3-modified Ag nanoparticles for industrial applications?
Scalability relies on improving synthesis techniques and tackling economic problems related to the production of large quantities.
For more chemistry blogs, visit chemistry Mater