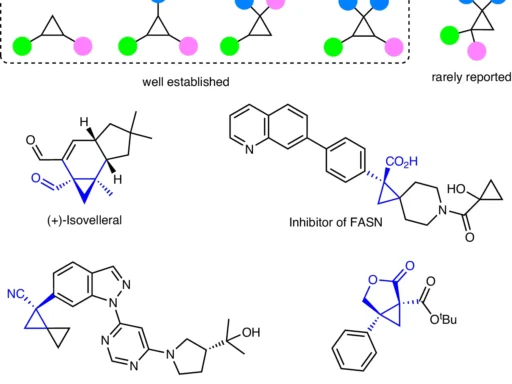
Table of Contents
Overview of Continuous Flow Chlorination:
Electrocatalysis is a cutting-edge discipline that utilizes electrical energy to facilitate chemical processes, leading to improved efficiency and selectivity. Chlorination is an essential technique for introducing chlorine into organic molecules, which is crucial for the production of numerous useful chemicals. This article talks about the advanced method of electrocatalytic continuous flow chlorinations and how important iodine (I/III) mediators are.
Historical Background of Chlorination:
Historical Approaches to Chlorination:
Chlorination has been a crucial procedure in chemical synthesis for more than a century. At first, it entailed the utilization of chlorine gas, which is an extremely poisonous and corrosive material. While this approach was indeed efficient, it presented substantial hazards to both the ecosystem and human well-being. Despite their effectiveness, the early systems necessitated rigorous safety precautions and generated significant waste, rendering them less acceptable.
Transition to contemporary methods:
Over time, progress in the field of chemistry resulted in the development of chlorination processes that were both safer and more effective. Scientists developed reagents like sulfuryl chloride and hypochlorites to replace chlorine gas. These techniques were easier to use and provided superior command of the reaction conditions. Nevertheless, the demand for procedures that are even more secure, environmentally friendly, and effective continued, leading to the emergence of electrocatalytic technologies.
Significant advancements in electrocatalytic techniques:
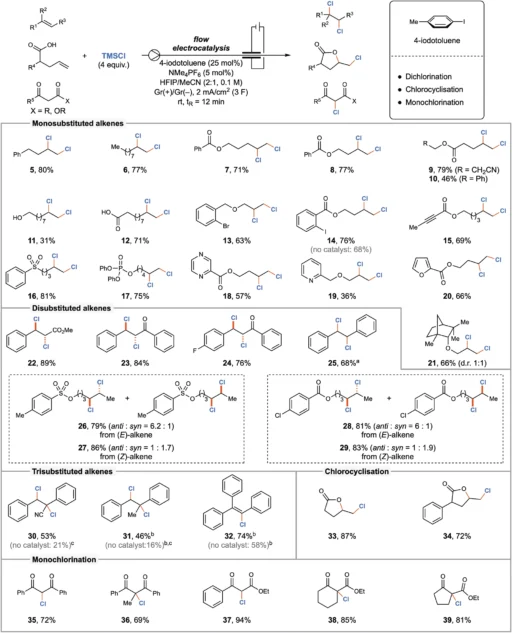
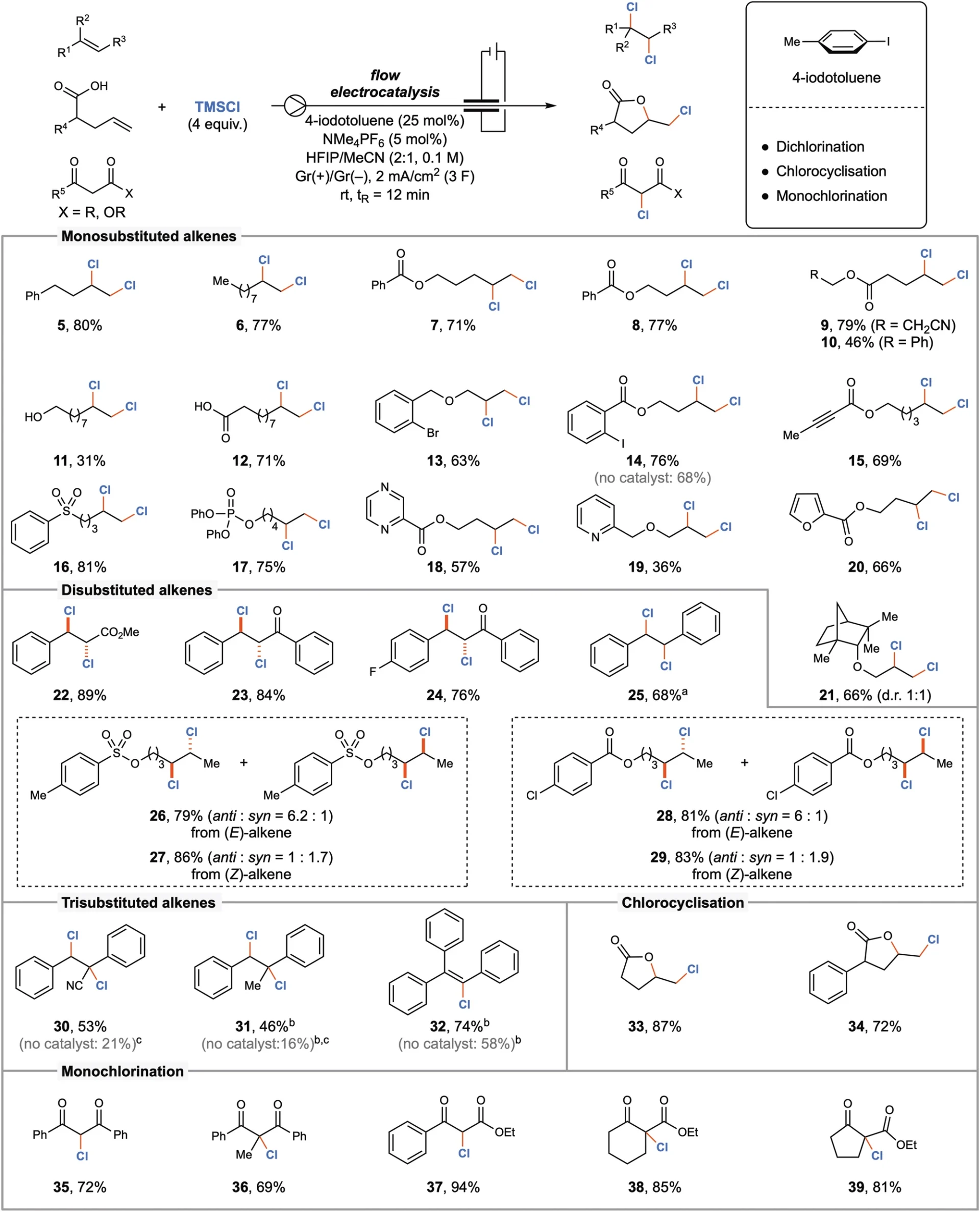
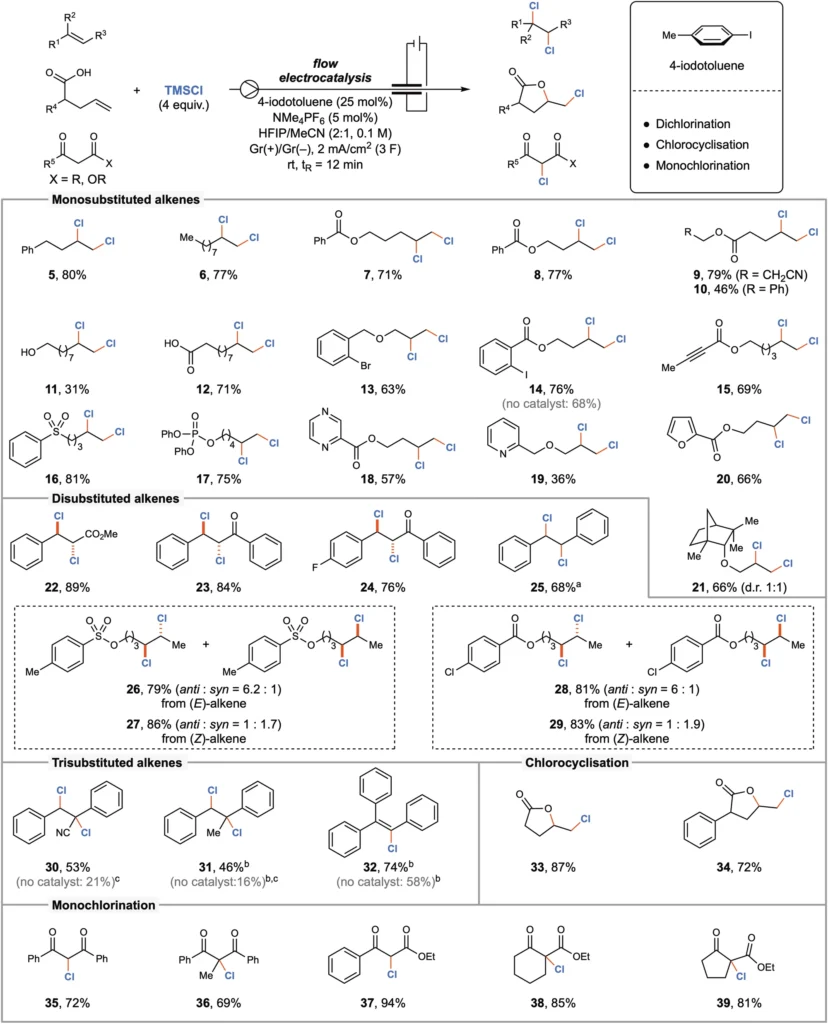
The introduction of electrocatalysis marked a significant milestone in the development of chlorination techniques. Electrocatalytic techniques employ electrical energy to facilitate chemical reactions, obviating the requirement for dangerous reagents. This strategy not only increases safety but also promotes reaction efficiency and selectivity. By using iodine (I/III) mediators, these processes have been further enhanced, resulting in electrocatalytic chlorination being a valuable tool in contemporary organic synthesis. Scope of the electrocatalyzed chlorination reaction.
An Explanation of Continuous Flow Chemistry:
Introduction to Continuous Flow Chemistry:
Continuous flow chlorination chemistry is a method that involves the uninterrupted circulation of reactants through a reactor, in contrast to the conventional batch processing approach, where reactants are mixed in a single container. Continuous flow systems continuously introduce reactants into the reactor and consistently extract products. This approach provides meticulous regulation of reaction conditions, resulting in enhanced reaction efficiency and product quality.
Contrast with batch processing:
Batch processing, which involves combining and reacting reactants in a single batch, has traditionally been the preferred method in chemical synthesis. Although it is efficient, it frequently leads to inconsistent product quality and decreased efficiency. Continuous flow chemistry, in contrast, enables enhanced regulation of reaction parameters such as temperature, pressure, and reaction time. This control results in increased product uniformity and enhanced production efficiency.
Benefits in Industrial Applications:
Continuous flow chemistry offers advantages that go beyond the confines of the laboratory and find practical use in commercial settings. Continuous flow technologies in industries such as pharmaceuticals and agrochemicals have the advantages of scalability, enhanced safety, and decreased production costs. Continuous monitoring and adjustment of reaction conditions guarantee enhanced quality and uniformity in the end products. Furthermore, the reduced response times and smaller reactor volumes result in substantial cost savings.
Electrocatalysis for Organic Synthesis:
Principles of Electrocatalysis:
Electrocatalysis is the process of utilizing electrical energy to facilitate chemical reactions on a catalyst’s surface. This technique can greatly reduce the energy threshold for reactions, resulting in increased efficiency and selectivity. Electrocatalysis in organic synthesis provides a more environmentally friendly and sustainable option than conventional techniques by decreasing reliance on dangerous substances and reducing trash generation.
Mechanisms and pathways:
The surface of the catalyst is essential to promoting electrocatalytic processes. The catalyst facilitates the transfer of electrons between the reactants, hence promoting the chemical reaction. The individual reactions and catalysts involved can cause variations in the processes and pathways. Gaining a comprehensive understanding of these mechanisms is crucial for fine-tuning reaction conditions and attaining the intended results. Mechanistic investigations.
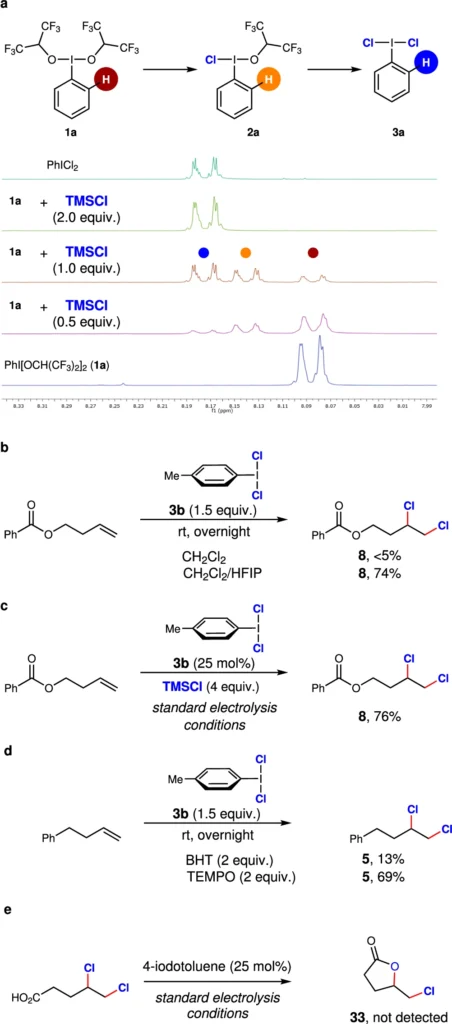
Advantages of organic reactions:
Electrocatalysis offers a multitude of advantages in organic processes. The following items are included:
Increased Efficiency: In comparison to conventional approaches, electrocatalytic reactions frequently exhibit enhanced reaction rates and higher product yields.
Enhanced Selectivity: The capacity to accurately manipulate reaction conditions results in increased selectivity, minimizing the production of undesired by-products.
Environmental Sustainability: Electrocatalysis provides a more environmentally sustainable method for chemical synthesis by decreasing the reliance on harmful chemicals and reducing waste production.
Chemical Characteristics of iodine (I) and iodine (III) :
Structural and chemical reactivity:
Iodine is found in several oxidation states, and iodine (I) and iodine (III) are especially valuable in chlorination reactions. Iodine (I) compounds, such as iodoarenes, and iodine (III) compounds, like iodosylbenzenes, possess distinct chemical characteristics that contribute to their efficacy as mediators.
Iodine (I): molecules, which are generally less reactive, serve as intermediates in the creation of more reactive iodine (III) species.
Iodine (III): compounds are extremely reactive and can transfer chlorine atoms to organic substrates, hence aiding chlorination.
Role in Facilitating Chlorination:
Iodine (I/III) mediators are essential in electrocatalytic continuous flow chlorination because they facilitate chlorine atom transport. Iodine facilitates the effective production and transmission of reactive chlorine species, which then react with the organic substrate to achieve targeted chlorination. The mediation process improves the efficiency and selectivity of reactions, making it a highly valuable tool in organic synthesis.
Comparative Analysis of Halogens:
Iodine presents distinct advantages in chlorination reactions when compared to other halogens such as chlorine, bromine, and fluorine. Its capacity to exist in several oxidation states and its reactivity characteristics make it especially well-suited for facilitating chlorination. In comparison to other halogens, iodine’s larger atomic size and lower electronegativity enhance its efficacy as a mediator by allowing for more specific reactions.
Explanation of Iodine (I/III) Facilitated Chlorinations:
We provide sequential and detailed explanations of mechanistic pathways:
The process of iodine-mediated chlorination consists of several critical stages:
a). The generation of reactive iodine species initiates with the oxidation of iodine (I) molecules to form iodine (III) species. Usually, we use an external oxidant or an electrochemical technique to facilitate this phase.
b). Formation of Chlorine-Transfer Species: The iodine (III) species reacts with a source of chlorine to create a chlorine-transfer species that is extremely reactive.
c). Chlorination of Substrate: When you chlorinate a substrate, a chlorine-transfer species reacts with the organic substrate. This causes a chlorine atom to be transferred, which is what chlorination is all about.
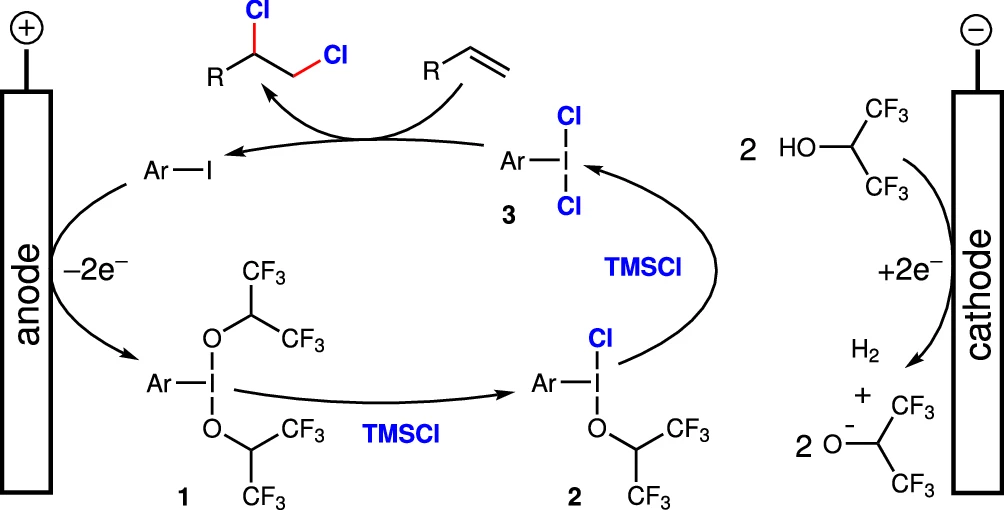
Substrate Interaction:
The successful chlorination reaction heavily relies on the crucial interaction between the iodine (III) species and the organic substrate. The reactivity and selectivity of this interaction are determined by a variety of factors, including the substrate’s characteristics and the specific iodine (III) molecule used. Understanding these interactions is critical for optimizing reaction conditions and achieving high yields and selectivity.
Function in Electrocatalysis:
Iodine (I/III) mediators help chlorine atoms move more efficiently when there is an electric current present in electrocatalytic chlorination. The electrocatalytic technique allows for precise manipulation of the production and movement of reactive species, thereby improving the overall efficiency and selectivity of the chlorination reaction.
Experimental configuration for continuous flow chlorination:
Indispensable Gear and Configuration:
A standard continuous-flow chlorination arrangement has multiple essential components:
1). The flow reactor is the central component of the system, where the chlorination reaction takes place. Flow reactors exhibit diverse designs, encompassing microreactors, tubular reactors, and packed-bed reactors.
2). Pumps: Reactants enter the reactor through pumps. Accurate pumps guarantee a uniform rate of flow, which is essential for preserving reaction conditions.
3). An electrochemical cell is critical for electrocatalytic reactions because it provides the necessary electrical energy to facilitate the reaction.
4). Control System: To maintain ideal reaction conditions, automated control systems oversee and regulate reaction parameters, such as temperature, pressure, and flow rates.
Sequential Process:
1). We produce the reactants, which include the organic substrate and iodine (I/III) mediator, and then dissolve them in a suitable solvent.
2). Installation of the Flow Reactor: We have installed the flow reactor and introduced the reactants into the system.
3). Reaction Initiation: We trigger the pumps and introduce the reactants into the flow reactor. We also stimulate the electrochemical cell to produce the necessary electrical energy.
4). Monitoring and Adjustment: We constantly monitor and modify reaction parameters to maintain the most favorable conditions.
5). Product Collection: The reactor consistently collects the chlorinated product at the exit.
Safety Consideration:
Ensuring safety is of utmost importance in continuous-flow chlorination systems. The following are crucial factors to consider for safety:
1). Proper ventilation: means ensuring sufficient airflow to prevent the buildup of noxious gases.
2). Chemical Management: Appropriate management and storage of reactive chemicals, such as iodine compounds and chlorine sources.
3). Electrical Safety: To prevent electrical hazards, make sure the electrochemical cell and its related electrical components are sufficiently insulated and connected to the ground.
Enhancement of reaction conditions:
Factors Affecting Chlorination:
Several factors, including the following, can influence the outcome of a chlorination reaction.
1). Reactant Concentration: The organic substrate and iodine (I/III) mediator concentration can have a significant effect on the reaction’s efficiency and selectivity.
2). Flow rates: The speed at which reactants enter the flow reactor affects both the length of the reaction and the volume of product generated.
3). The temperature and pressure of the reaction can influence the reactivity of iodine species and the total reaction rate.
4). In electrocatalytic processes, the electric potential is a critical factor because it drives the reaction and produces reactive species.
Optimization Techniques:
Achieving optimal response conditions necessitates a methodical approach. We can use the Design of Experiments (DoE) to study the impact of different factors and determine the best circumstances. This strategy encompasses:
1). Setting the Objective: Precisely establishing the intended result, such as achieving the highest possible yield or selectivity.
2). Experiment Design: Develop a set of experiments to methodically manipulate response conditions and observe the resulting outcomes.
3). Data Analysis: Examining the experimental data to detect patterns and ascertain the most favorable conditions.
4). Validating Results: performing further experiments to confirm the optimal settings and guarantee reproducibility.
Comprehensive Case Studies:
Case studies offer significant insights into the process of optimization. An example of this could be a study examining the chlorination process of a specific organic substrate, which could include:
1). Initial Screening: Conduct preliminary studies to assess the efficacy of iodine (I/III) mediators and identify critical factors.
2). Optimization experiments involve deliberately altering reaction parameters, such as the concentrations of reactants, rates of flow, and temperature, systematically to determine the most favorable conditions.
3). Validation and Scale-Up: The process of confirming the optimum conditions by conducting supplementary experiments and expanding the process for larger-scale manufacturing. Aryl iodides in electrochemistry.
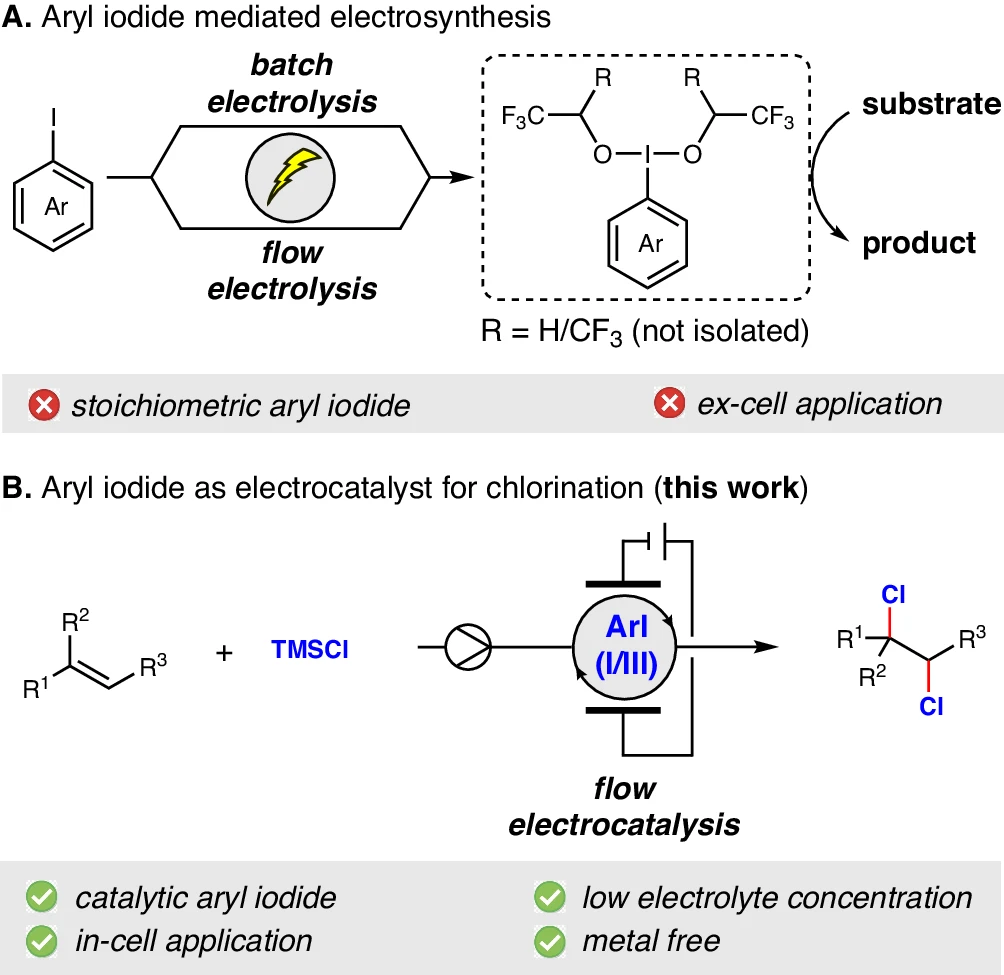
Comparative analysis of catalytic methods:
Comparison between Traditional and Electrocatalytic Chlorination:
Conventional chlorination processes sometimes utilize dangerous substances and severe reaction conditions. On the other hand, electrocatalytic chlorination presents numerous benefits:
Safety: Electrocatalytic techniques eliminate the need to use perilous chlorine gas, thereby reducing safety hazards.
Efficiency: Using electrical energy and iodine (I/III) mediators enhances reaction efficiency, increasing yields and reducing by-product production.
Environmental Impact: Electrocatalytic chlorination has a lower environmental impact compared to other methods. It produces fewer harmful by-products and reduces waste, making it a more ecologically friendly choice.
The study compares Iodine (I/III) mediators with other catalysts:
Iodine (I/III) mediators have distinct benefits in chlorination processes when compared to other catalysts.
1). Selectivity: Iodine (I/III) mediators exhibit exceptional selectivity, guaranteeing that chlorination takes place precisely at the intended location on the substrate.
2). Reactivity: Iodine’s capability to exist in several oxidation states enables the effective production and transmission of highly reactive chlorine species.
3). Versatility: Iodine (I/III) mediators exhibit broad applicability in various chlorination processes, making them highly adaptable instruments in organic synthesis.
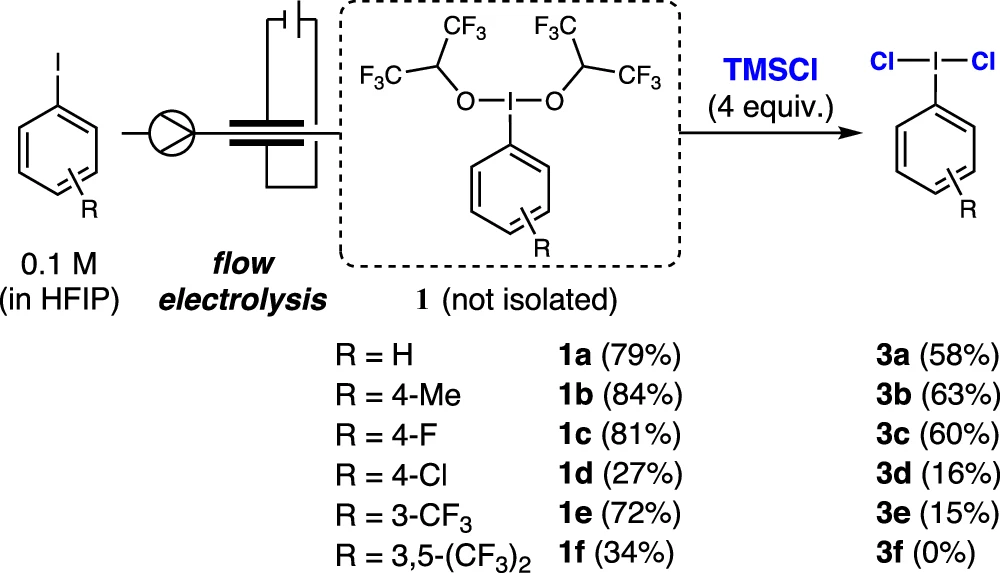
Advantages and Disadvantages: Electrocatalytic chlorination using iodine (I/III) mediators provides some advantages, but it is also subject to certain limitations:
Benefits:
There has been an improvement in safety and an increased focus on environmental sustainability.
a). Increased efficiency and selection.
b). Several chlorination reactions are flexible.
Drawbacks:
Technical difficulties encountered when establishing and sustaining continuous flow systems.
a). The company is investing in equipment and optimizing processes.
b). Utilization in Diverse Sectors
Applications in Various Industries:
Pharmaceutical sector: Chlorinated chemicals are essential in the pharmaceutical sector for drug creation. Using electrocatalytic continuous flow chlorination is a reliable and efficient way to make these chemicals that are very pure and uniform. This method is extremely useful in the production of active pharmaceutical ingredients (APIs) and intricate medicinal compounds.
Agrochemicals:
The agrochemical sector depends on chlorinated chemicals for the manufacturing of insecticides and herbicides. Continuous flow chlorination offers a scalable and cost-effective approach for manufacturing these chemicals. The process’s great selectivity and efficiency guarantee the acquisition of required products with few contaminants, hence improving the effectiveness and safety of the final agrochemical products.
Study of the properties, structure, and behavior of materials.
Continuous flow chlorination electrocatalysis provides precision control that is advantageous for chlorinated polymers and other materials. This method enables the production of superior materials with customized characteristics, which are crucial in diverse applications such as coatings, adhesives, and electronic materials. The capacity to consistently generate these materials also facilitates extensive manufacturing and industrial uses.
Illustrative Analyses and Practical Instances:
Various companies have successfully applied electrocatalytic continuous flow chlorination, as demonstrated by several case studies.
Pharmaceuticals: The process of creating a chlorinated Active Pharmaceutical Ingredient (API) was improved by employing iodine(I/III) mediators, resulting in a substantial enhancement in both the amount and quality of the final product. The implementation of the continuous flow process facilitated the ability to produce goods on a larger scale, hence satisfying the requirements of large-scale manufacturing.
Agrochemicals: A method for producing a crucial pesticide component was devised using a continuous flow chlorination process in the field of agrochemicals. The revised process achieved a high degree of selectivity and minimized the production of undesired by-products, hence enhancing the overall efficiency and cost-effectiveness.
Material Science: The technique of continuous flow electrocatalysis was employed to produce a chlorinated polymer with tailored characteristics suitable for electronic materials. The process ensured consistent product quality and enabled efficient mass production.
Obstacles and Constraints:
Technical obstacles:
The execution of continuous flow chlorination operations presents numerous technological obstacles:
Equipment Setup: The process of establishing and sustaining continuous flow systems necessitates specific expertise and specialized equipment. It is essential to ensure the effective integration and functioning of all components, such as the flow reactor, pumps, and electrochemical cell, for successful operation.
Reaction Control: Precise control of reaction conditions, including flow rates, temperature, and electric potential, is crucial for producing the intended consequences. Any departure from ideal conditions can affect the effectiveness of the reaction and the quality of the result.
Financial Factors:
Although continuous-flow chlorination provides economic benefits in the long run, the initial outlay for equipment and optimization might be substantial. Small-scale laboratories and organizations may encounter budgetary obstacles while implementing this technology. In addition, it is important to take into account the continuous expenses associated with maintenance and operation when assessing the overall economic viability.
Possible resolutions:
Continuing research and development endeavors are focused on tackling these challenges:
User-Friendly Equipment: Developing equipment that is both user-friendly and cost-effective for continuous flow chlorination can reduce the obstacles to its adoption. Streamlining the configuration and functionality of these systems can enhance the accessibility of the technology to a wider spectrum of users.
Scalable Processes: Developing scalable techniques that can be readily adjusted to various production sizes can improve the flexibility and economic feasibility of continuous flow chlorination. This technique guarantees the applicability of the technology in both small-scale laboratory settings and large-scale industrial operations.
Latest Progress & Breakthroughs:
Iodine(I/III) mediators in a novel form:
Current studies have concentrated on creating new iodine(I/III) mediators that have improved performance. These newly developed mediators exhibit enhanced responsiveness, specificity, and durability, rendering them more potent in electrocatalytic chlorination processes. Progress in comprehending the connections between the structure and activity of these agents has also played a role in improving and utilizing them in different reactions.
Advancements in technology:
The efficiency and scalability of continuous flow chlorination procedures have been greatly enhanced by technological improvements. Advancements in flow reactor design, automation, and process control have improved the overall efficiency and dependability of these systems. By incorporating sophisticated monitoring and feedback systems, it becomes possible to continuously optimize reaction conditions in real-time, thereby guaranteeing consistent product quality and yield.
Noteworthy Research Discoveries:
A recent study has emphasized the potential of electrocatalytic continuous flow chlorination in many applications. Notable discoveries consist of:
1). Increased Efficiency: Research has shown that electrocatalytic processes can achieve more efficiency and selectivity when compared to conventional approaches, resulting in shorter reaction times and higher yields.
2). Environmental Advantages: Studies have demonstrated that the process of continuous flow chlorination produces fewer harmful by-products and minimizes waste, promoting a more sustainable approach to chemical synthesis.
3). Flexibility: The adaptability of iodine(I/III) mediators has been demonstrated in diverse chlorination reactions, underscoring their promise in several domains of chemical synthesis.
Prospects for the future of electrocatalytic Continuous Flow chlorination:
Current and developing patterns and advancements in technology:
Various upcoming developments and technologies are anticipated to influence the future of electrocatalytic continuous flow chlorination:
a). Integration with Green Chemistry: The incorporation of green chemistry principles, such as the utilization of renewable energy sources and sustainable reagents, is anticipated to improve the environmental sustainability of continuous flow chlorination processes.
b). Advanced Materials: The advancement of materials for flow reactors and electrochemical cells has the potential to enhance the efficiency and longevity of these systems. Nanocatalysts, among other catalyst materials, have the potential to improve reaction performance through innovative advancements.
c). Digitalization and Automation: The implementation of digitalization and automation in continuous flow systems can enhance process control and optimization. Artificial intelligence and machine learning algorithms can be employed to continuously monitor and modify reaction conditions, thereby guaranteeing consistent product quality and yield in real time.
Possible avenues for future research:
Future investigations in electrocatalytic continuous flow chlorination are expected to concentrate on four pivotal domains:
Mechanistic Studies: Mechanistic studies entail a thorough examination of the pathways and intermediates involved in iodine(I/III) mediated chlorinations. These studies aim to get a better understanding of the process, which may then be used to improve reaction conditions and discover new mediators.
Process Optimization: Process optimization involves researching to improve the efficiency and selectivity of continuous flow chlorination processes by adjusting reaction parameters, including temperature, pressure, and electric potential. Creating reliable optimization techniques can help promote the broad use of this technology.
Scalable Applications: Investigating the potential uses of continuous flow chlorination in different sectors can showcase the adaptability and cost-effectiveness of this technology. Case studies and real-world examples offer useful insights that can be utilized for industrial adoption.
Potential for Industry Adoption:
As the electrocatalytic continuous flow chlorination technology becomes more accessible and cost-effective, its usage in industry is anticipated to rise. Multiple variables contribute to this pattern:
Environmental Regulations: The use of cleaner and greener chemical synthesis technologies is driven by stricter environmental restrictions and an increasing focus on sustainability. Electrocatalytic continuous flow chlorination provides a more sustainable option compared to conventional technologies, in line with industrial objectives for environmental adherence.
Economic advantages: The enduring financial savings and enhanced effectiveness of continuous flow processes render them appealing to enterprises aiming to maximize production and minimize operational expenses. The economic viability of these methods is further enhanced by their scalability and adaptability.
Technological Advancements: Technological developments in flow reactor design, automation, and process control are constantly improving the performance and reliability of continuous flow chlorination systems. These advancements enhance the functionality and availability of the technology for industrial use. Preliminary studies for enantioselective variant.
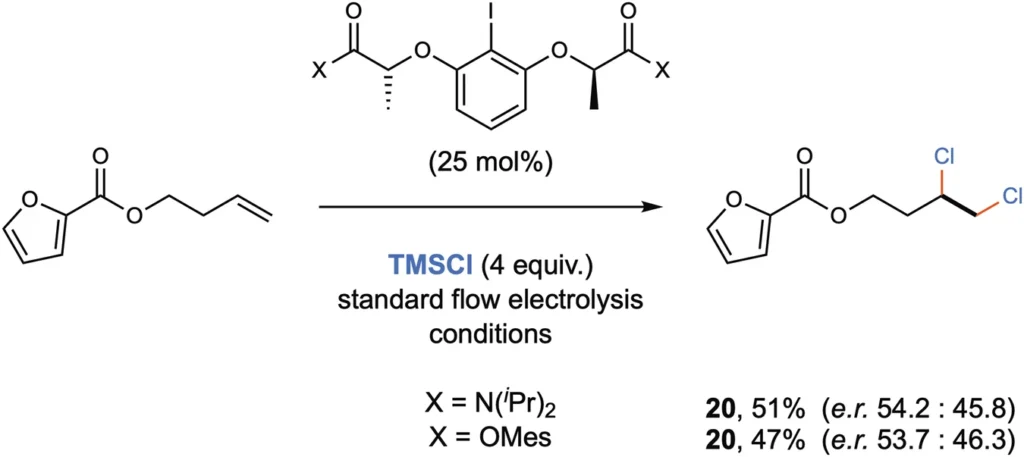
Environmental and safety factors:
Efficient Management and Minimization of Waste:
Continuous flow chlorination technologies provide substantial environmental advantages by decreasing waste and eliminating the production of dangerous by-products. Exerting precise control over the conditions in which a reaction takes place and making optimal use of the substances involved result in increased production and decreased waste. Moreover, the uninterrupted characteristic of the process enables more effective control of reaction by-products and waste streams.
Regulatory Aspects:
Adherence to environmental standards is an essential factor to consider in chemical synthesis. Continuous flow chlorination processes comply with regulatory standards by providing safer and more environmentally friendly alternatives to conventional methods. Minimizing the utilization of dangerous substances and the production of a smaller amount of harmful by-products result in a decreased environmental footprint and enhanced adherence to regulatory requirements.
Sustainable Practices: Practices that are environmentally friendly and can be maintained over a long time.
Implementing sustainable techniques in continuous flow chlorination encompasses various crucial elements:
a). Resource optimization involves the effective utilization of reactants and energy sources to limit resource consumption and decrease the overall environmental impact of the process.
b). Green Chemistry Principles: By implementing green chemistry principles, such as utilizing renewable energy and sustainable reagents, the sustainability of continuous flow chlorination processes is improved.
c). Life Cycle Assessment (LCA): By conducting life cycle assessments, we may analyze the environmental impact of continuous flow chlorination operations. This evaluation can provide valuable insights for optimizing and improving these processes.
In conclusion:
Electrocatalytic continuous flow chlorinations using iodine(I/III) mediators are a notable progress in organic synthesis. This technique provides a multitude of benefits, such as enhanced selectivity, efficiency, and less environmental impact. By combining continuous flow chlorination chemistry with electrocatalysis, it is possible to achieve precise manipulation of reaction conditions, resulting in improved performance and scalability of chlorination processes. With ongoing research and development, the utilization of this technology is projected to increase, leading to the implementation of more environmentally friendly and economically efficient chemical processes.
Frequently Asked Questions:
1. Iodine(I/III) mediators are substances that facilitate chemical reactions by transferring iodine atoms between reactants.
Iodine(I/III) mediators are chemical substances that aid chlorination processes by transferring chlorine atoms, hence improving the efficiency and selectivity of the process.
2. What are the distinctions between continuous flow chemistry and batch processing?
Continuous flow chemistry entails the uninterrupted flow of reactants through a reactor, providing enhanced control overreactions, safety, and scalability in comparison to conventional batch processing.
3. What are the primary benefits of electrocatalytic continuous flow chlorination?
Electrocatalytic continuous flow chlorination offers superior selectivity, efficiency, and environmental advantages in comparison to conventional techniques, resulting in decreased production of hazardous waste and enhanced reaction results.
4. Do iodine(I/III) mediators have any environmental advantages?
Iodine(I/III) mediators enhance the efficiency of the process by minimizing the production of harmful by-products and decreasing the use of dangerous chemicals, so promoting a more ecologically sustainable approach.
5. Which industry experiences the greatest advantages from this technology?
Electrocatalytic continuous flow chlorination is highly advantageous for industries including pharmaceuticals, agrochemicals, and material science. This method offers manufactured molecules that are exceptionally pure and efficient, resulting in major benefits for these industries.
For more chemistry blogs, visit chemistry Master