Table of Contents
Overview of regio-divergent hydrosilylation:
Adding silicon-hydrogen (Si-H) bonds to unsaturated bonds, like those in alkenes and alkynes, is a key step in organic chemistry. The production of organosilicon compounds, used in various industries such as medicine, materials science, and agrochemicals, fundamentally relies on this reaction. To make specific molecules, it is important to have precise control over the regio-divergent hydrosilylation. This refers to where the silicon atom attaches to the organic molecule. Regio-divergent hydrosilylation, which selectively produces various regioisomers from a common starting material, enhances this procedure’s synthetic versatility.
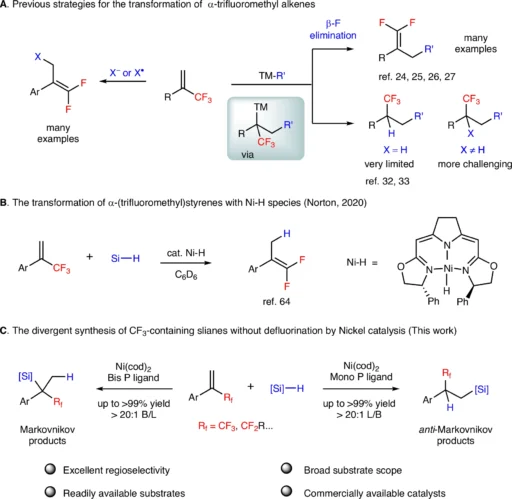
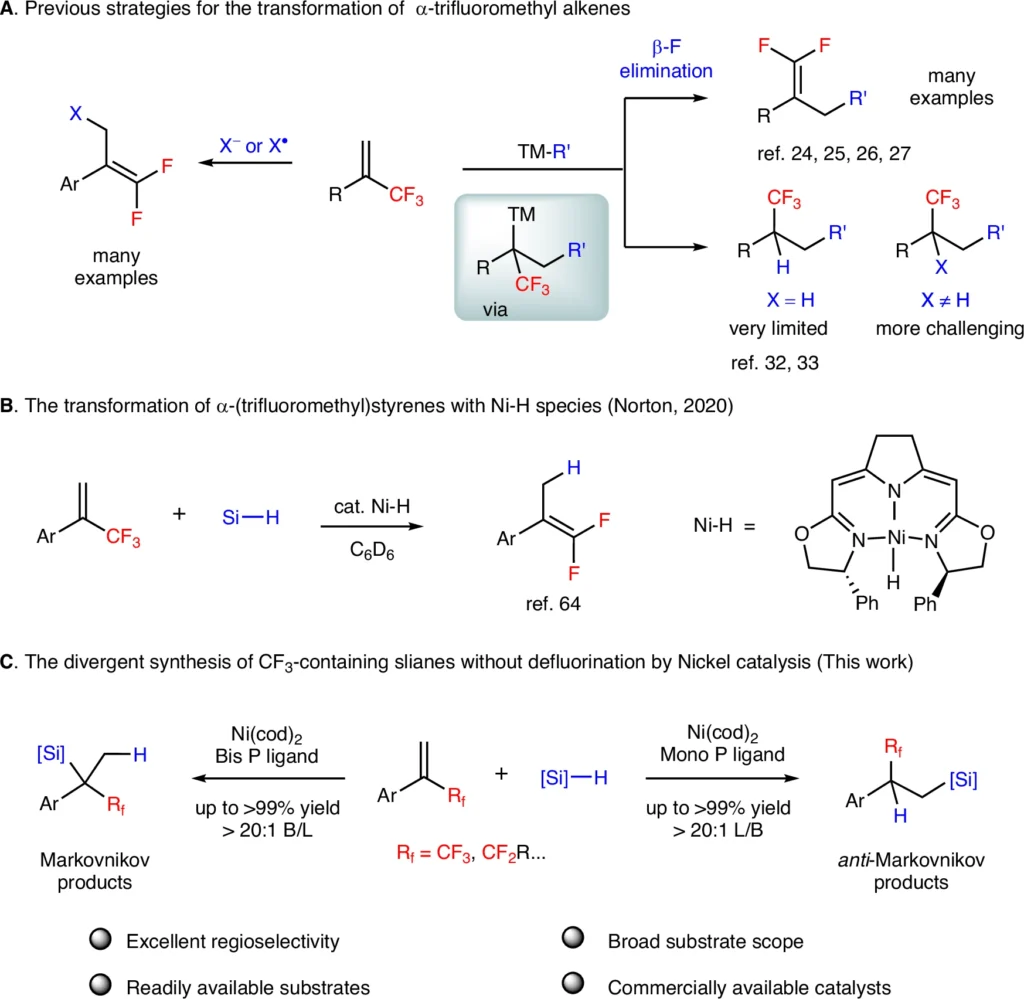
α-(fluoroalkyl)styrenes are fascinating compounds for regio-divergent hydrosilylation reactions because of their distinctive electrical characteristics and widespread use in pharmaceuticals and other sophisticated materials. Nevertheless, altering these compounds without defluorination (removing fluorine atoms) poses significant difficulties. This article talks about how the nickel-catalyzed regio divergent hydrosilylation of α-(fluoroalkyl)styrenes works, how the experiments were done, the results, and what these results mean for organic synthesis as a whole. The transformation of α-(trifluoromethyl)styrenes by transition-metal catalysis.
Introduction to regio-divergent Hydrosilylation Fundamentals:
Hydrosilylation, which was first identified in the 1950s, is the process of adding a silicon-hydrogen bond to multiple carbon-carbon bonds. A metal catalyst often facilitates efficient progress in this reaction. We can break down the fundamental process of hydrosilylation into several crucial stages:
Activation of the Catalyst: A process that involves the formation of a reactive species activates the catalyst. This catalyst is often a transition metal.
Alkene/alkyne coordination: The substrate with double or triple bonds attaches to the metal center.
Insertion of the Si-H Bond: The metal-carbon bond incorporates the Si-H bond.
Product Formation: We discharge the ultimate product and rejuvenate the catalyst.
Regio-divergent Hydrosilylation:
The chemical process known as hydrosilylation involves the addition of a silicon hydride compound to an unsaturated organic molecule in a manner that leads to the
Regioselectivity is the tendency to favor the formation of one specific constitutional isomer over another in a chemical process. Hydrosilylation is the process of forming either the anti-Markovnikov or Markovnikov product, in which the silicon atom adds to distinct locations on the carbon-carbon double bond. Regio-divergent hydrosilylation allows for the targeted synthesis of various regioisomers from a single substrate by manipulating reaction conditions and catalyst properties. This is very advantageous for creating intricate molecules with exact structural specifications.
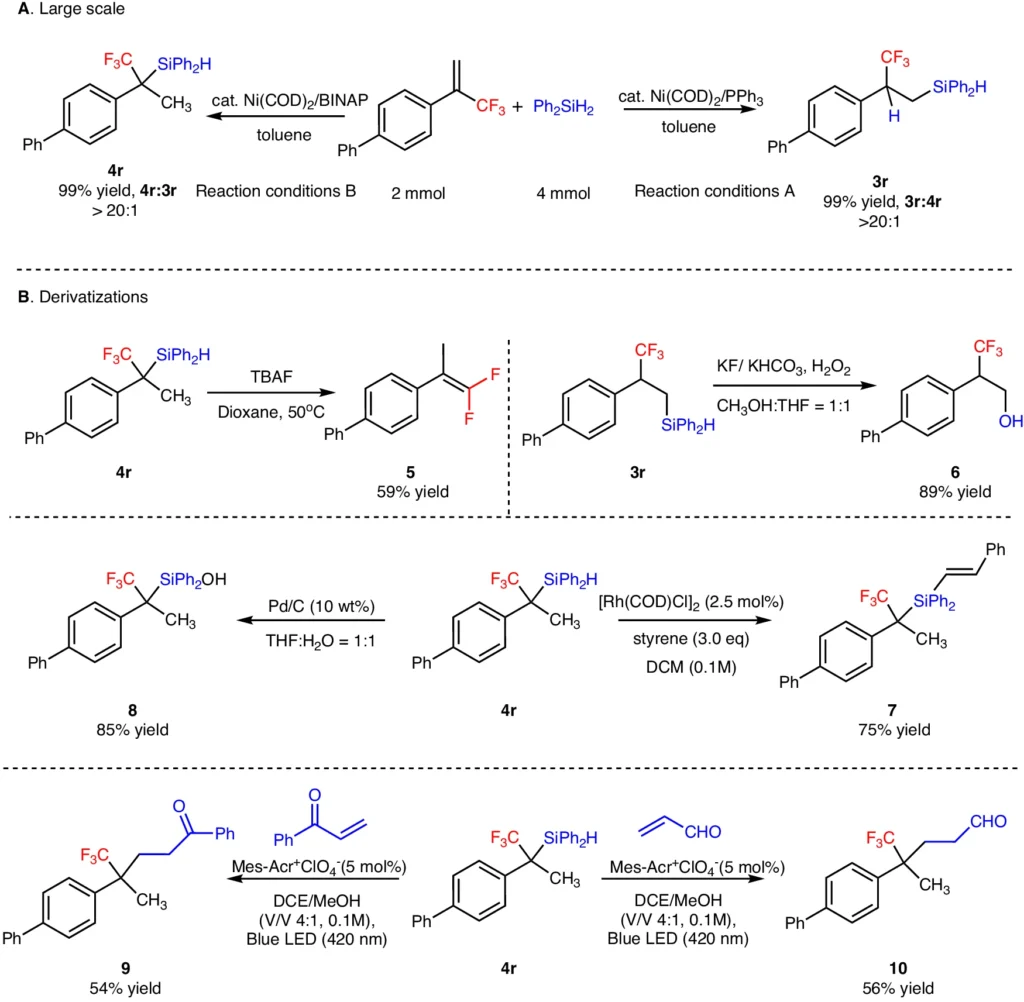
Attaining regio-divergent hydrosilylation presents a challenge because the substrate and catalyst have an innate tendency to prefer one pathway over another. To make precise adjustments, it is critical to have a profound understanding of how the catalyst behaves and the conditions in which the reaction takes place. Given its diverse electrical characteristics, nickel presents a possible answer to this challenge. Scale-up reaction and derivatization.
The use of nickel as a catalyst in organic reactions:
Researchers have extensively studied nickel, a plentiful and affordable transition metal, as a catalyst in numerous organic reactions. Its capacity to assume many oxidation states and bind with diverse ligands renders it extremely adaptable. Many processes, including cross-coupling, reduction, and hydrosilylation, have effectively utilized nickel catalysts.
The advantages of applying nickel catalysis are as follows:
Cost-effectiveness: Nickel is more economical compared to several other transition metals employed in catalysis, such as palladium and platinum.
Environmental sustainability: Nickel catalysts are frequently characterized by lower toxicity and greater sustainability.
High Efficiency: Nickel exhibits excellent efficiency by enabling reactions with high turnover numbers and selectivity.
α-(Fluoroalkyl)styrenes: Unique Characteristics:
Chemical compounds known as (Fluoroalkyl)styrenes form when a fluoroalkyl group bonds to the alpha position of a styrene molecule. These substances are highly intriguing because of their distinctive chemical properties:
Electronic Effects: Because these compounds contain a fluorine atom, it has strong electronic effects that make them very reactive and selective in certain processes.
Steric Effects: The fluoroalkyl group causes steric hindrance, which affects processes’ regioselectivity.
Applications: Because of their durability and biological activity, these compounds have utility in the fields of medicine, agrochemicals, and materials science.
Nevertheless, it is difficult to alter α-(fluoroalkyl)styrene while retaining the fluorine atom (defluorination). Fluorine’s high affinity for carbon and electronegativity may result in undesirable side reactions.
Mechanism of Nickel-Catalyzed Regio-divergent Hydrosilylation:
Here is a description of the nickel-catalyzed regio-divergent hydrosilylation of -(fluoroalkyl)styrene:
Catalyst Activation: During the reaction conditions, the nickel catalyst, often in the form of a complex like NiCl2 with a ligand, undergoes activation to generate a highly reactive nickel species.
Coordinating the substrate: The α-(fluoroalkyl)styrene forms a coordination bond with the nickel core, which lines up the double bond in a good spot for the next reaction.
Si-H bond insertion: The silane reagent’s Si-H bond is inserted into the carbon-nickel bond, assisted by the electrical characteristics of the nickel catalyst.
Formation of the product: The silicon atom bonds to the carbon atom at a precise position about the fluoroalkyl group to produce the intended regioisomer. We renew the catalyst for subsequent reaction cycles.
Depending on the nickel catalyst, the ligands used, and the reaction conditions (such as temperature and solvent), the specific regioselectivity that is reached will vary. Mechanistic studies.
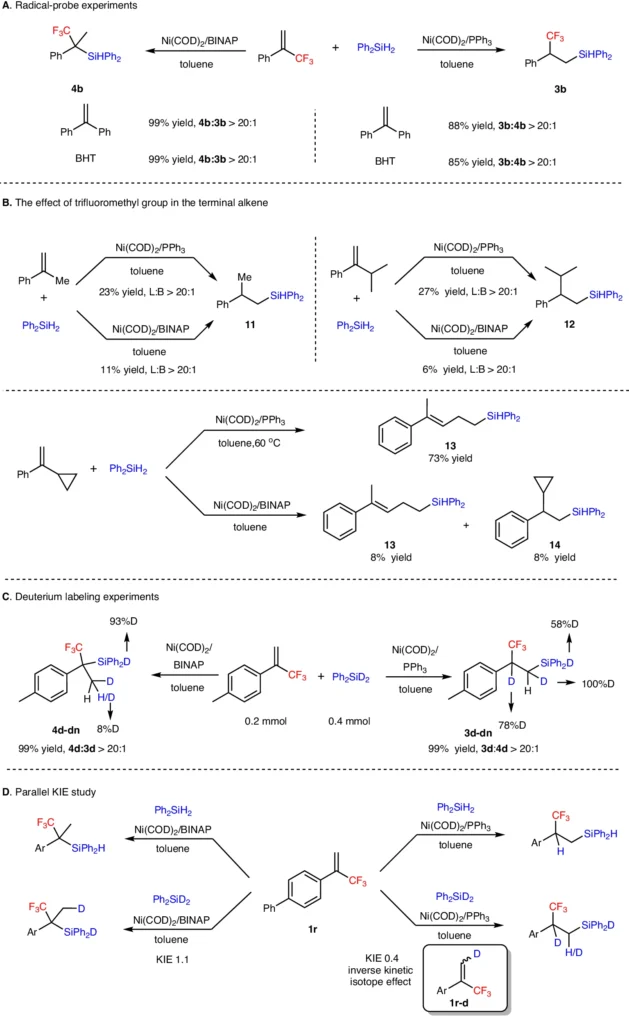
Methods used in scientific experiments:
The steps used in the experiments to study the nickel-catalyzed regio-divergent hydrosilylation of α-(fluoroalkyl)styrene include several important steps:
These are the things that are needed for the synthesis: very pure α-(fluoroalkyl)styrene, nickel catalysts like NiCl2, ligands like phosphines, and silane reagents like triethylsilane.
Experimental Procedure: To avoid oxidation of the catalyst and substrates, we typically perform the reaction in an environment free of reactive gases like nitrogen or argon.
Method: Dissolve the nickel catalyst and ligand completely in a suitable solvent like toluene or THF.
1). Introduce the α-(fluoroalkyl)styrene and silane reagents into the solution.
2). Agitate the concoction at a designated temperature range (e.g., 60–100 °C) for a predetermined duration (e.g., 12–24 hours).
3). Terminate the reaction and purify the resulting substances by chromatography.
Enhancement of reaction conditions:
Efficiently adjusting the reaction conditions is essential for attaining significant output and specificity. The following are important factors to consider:
Temperature: Elevated temperatures can enhance the rate of reaction, but they can also impact selectivity. Experimental methods are required to determine the most favorable temperatures.
Pressure: Although the majority of hydrosilylation processes are typically carried out at atmospheric pressure, certain reactions may be advantageously conducted at elevated pressures to enhance substrate solubility and reaction speeds.
Solvents: The choice of solvent can affect the reaction by altering the solubility of the reactants and the catalyst’s stability. Some commonly used solvents are toluene, tetrahydrofuran (THF), and dichloromethane.
Catalysts and ligands: The characteristics of the nickel catalyst and the ligands used can have a significant impact on the reaction’s selectivity and effectiveness. Various ligands can stabilize different nickel oxidation states, which in turn influences the course of the process.
Product analysis and characterization:
After the reaction has finished, it is necessary to evaluate and characterize the products to ascertain the distribution of regioisomers and assess their purity. The employed methods encompass:
We use nuclear magnetic resonance (NMR) spectroscopy to determine the precise structural characteristics of the goods, specifically the location of the silicon atom inside the carbon framework.
Scientists use gas chromatography-mass spectrometry (GC-MS) to separate and identify different compounds in a sample based on their physical and chemical properties. GC-MS analysis gives information about the substances’ molecular weight and make-up, which helps find different regioisomers.
High-Performance Liquid Chromatography (HPLC) serves as a valuable technique for separating and measuring substances, particularly in evaluating the ratio of regioisomers.
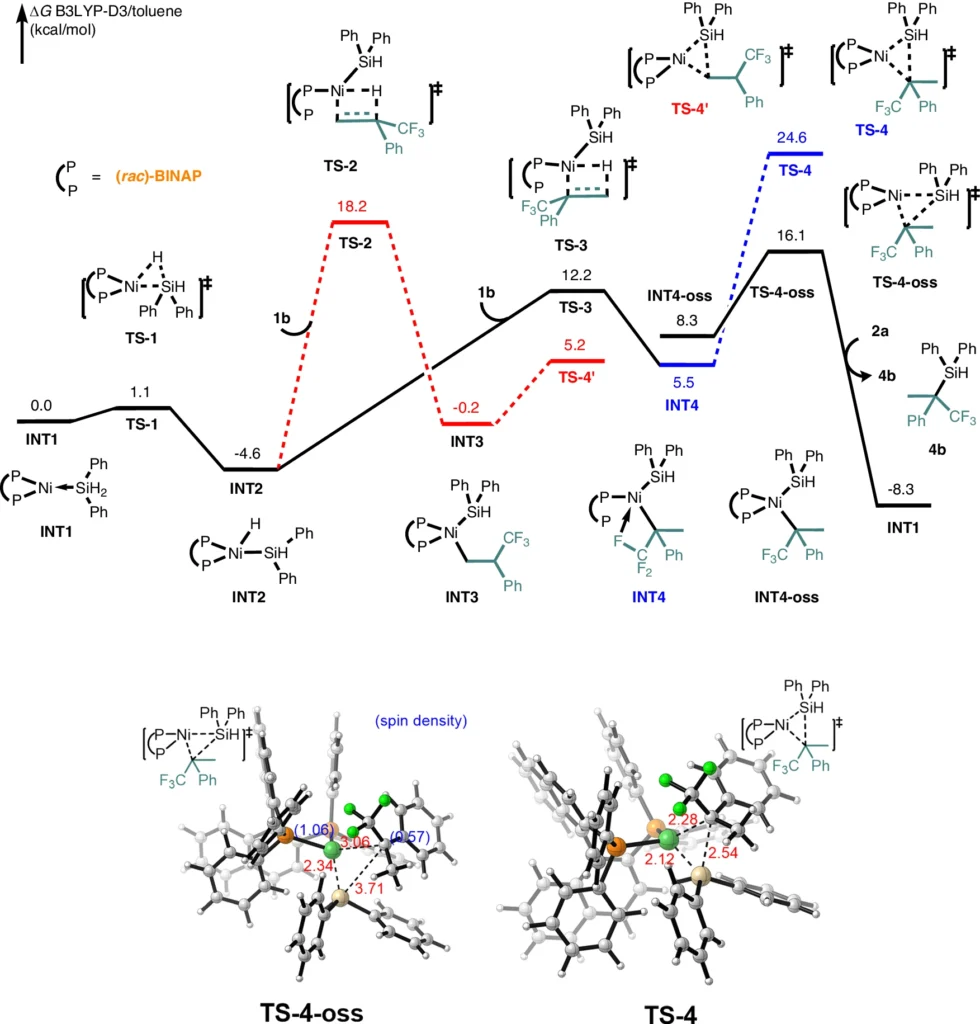
Elemental Analysis: This procedure verifies the existence and amount of elements in the product, guaranteeing that no defluorination has taken place. DFT Studies for Markovnikov product 4.
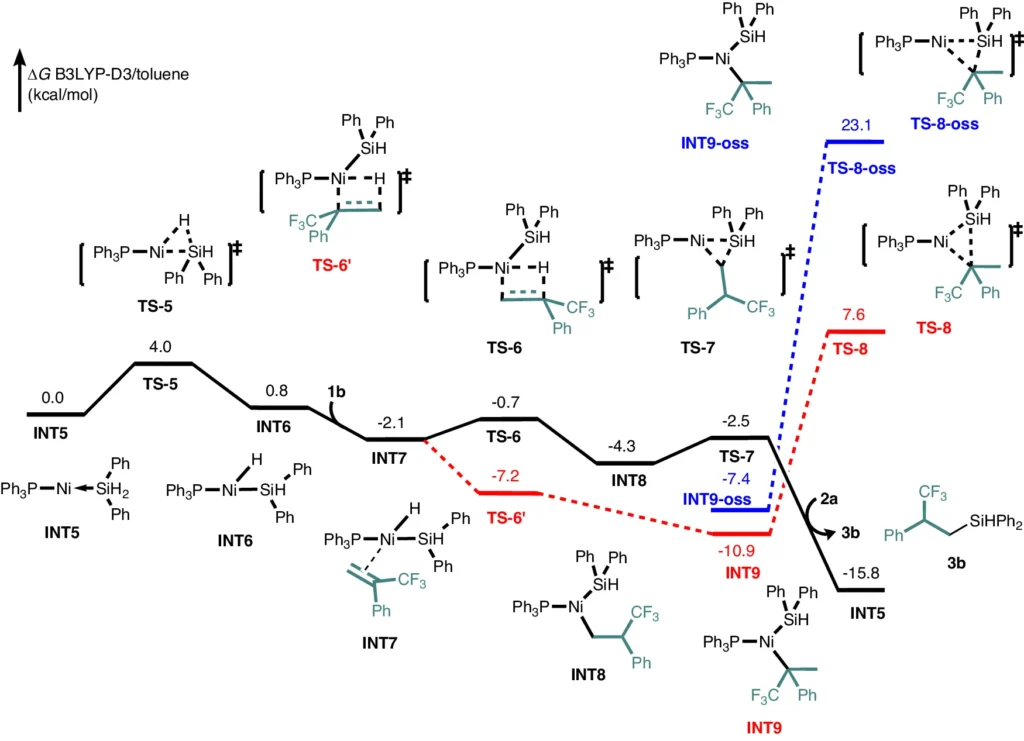
DFT Studies for anti-Markovnikov product 3.
Results and analysis:
The results of the experiments show that nickel catalysts can effectively help the regio divergent hydrosilylation of α-(fluoroalkyl)styrenes. Notable discoveries consist of:
High regioselectivity is the ability to selectively synthesize different regioisomers depending on the specific reaction conditions. The ligand selection and reaction temperature have a significant impact.
Minimal Defluorination: The process of reducing or minimizing the removal of fluorine atoms from a compound is referred to as minimal defluorination. This approach has the important benefit of retaining the fluorine atom, which is critical for preserving the desired characteristics of the product.
Comparative Analysis of Alternative Catalytic Systems: Nickel catalysts provide enhanced regioselectivity regulation in comparison to other transition metals such as palladium or platinum, which frequently result in defluorination or reduced selectivity.
Synthesis Applications:
The hydrosilylation of α-(fluoroalkyl)styrene using nickel as a catalyst yields compounds that have important practical uses.
Pharmaceuticals: The inclusion of the fluorine atom in these products improves their metabolic stability and bioavailability, making them highly useful in drug production.
Materials Science: We study the properties, structure, and performance of materials. We can use the obtained organosilicon compounds to produce sophisticated materials with precise electrical, optical, or mechanical characteristics.
Agrochemicals: Fluorine can enhance the effectiveness and environmental durability of these materials when used as intermediates in the production of agrochemicals.
Benefits of Nickel Catalysis:
Utilizing nickel catalysts in this process provides numerous benefits, including:
Economic efficiency: Due to its comparatively lower cost, nickel is well-suited for extensive utilization in industrial settings, particularly when compared to other transition metals.
Environmental Impact: Nickel exhibits low toxicity and possesses more environmental compatibility in comparison to denser metals such as platinum or palladium.
Efficiency: In hydrosilylation processes, nickel catalysts work especially well because they can achieve high regioselectivity and yield.
Obstacles and Constraints:
Although nickel-catalyzed hydrosilylation has certain benefits, it also encounters many obstacles:
Substrate Scope: It is important to note that not all α-(fluoroalkyl)styrenes are compatible with this reaction. Therefore, we need to expand the range of substrates suitable for this reaction.
Possible Secondary Reactions: Undesirable secondary reactions, such as defluorination or polymerization, may take place under specific circumstances.
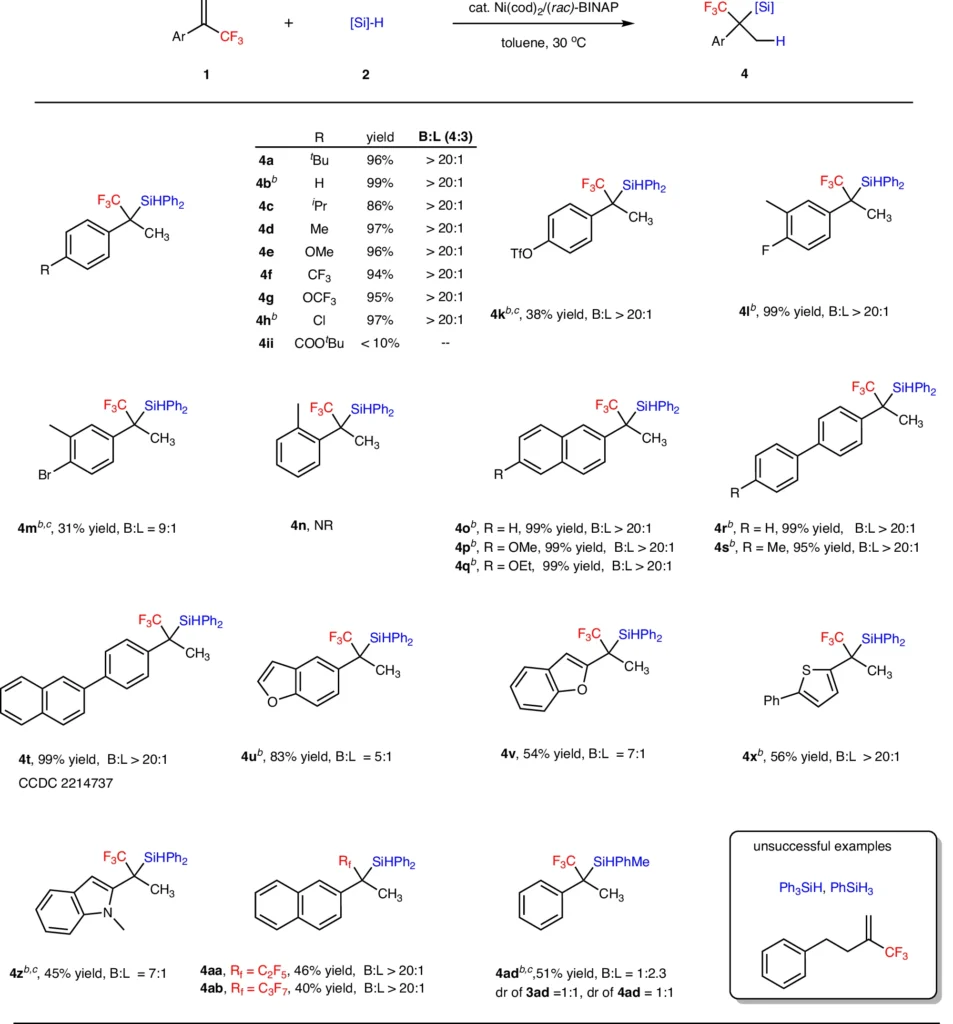
Catalyst Deactivation: Over time, the performance of nickel catalysts may diminish, necessitating the creation of more durable catalytic systems. Substrate scope for α-fluoroalkyl substituted silanes.
Prospects for the future:
Future research in the field of nickel-catalyzed hydrosilylation could focus on the following potential areas:
Catalyst advancement: Developing innovative nickel-based catalysts with improved durability and specificity.
Expansion of Substrate Scope: Investigating the reaction with a broader variety of fluoroalkylated compounds and other functionalized alkenes expands the range of substrates.
Synthetic integration refers to the process of combining hydrosilylation with other synthetic techniques to generate molecules that are more intricate and possess higher value.
In conclusion:
The use of nickel as a catalyst for the regio-divergent hydrosilylation of α-(fluoroalkyl)styrenes is a notable advancement in organic synthesis. This method is flexible and works well to make important organosilicon compounds that are selective for certain areas and lose as few fluorine atoms as possible. The advancement and fine-tuning of this reaction emphasize the capability of nickel catalysis to accomplish intricate synthetic conversions. Further research in this field will broaden its practicality and boost its usefulness in pharmaceuticals, materials science, and other areas.
Frequently Asked Questions:
1). Hydrosilylation is a chemical reaction that involves the addition of a silicon-hydrogen bond to a carbon-carbon double bond.
Adding silicon-hydrogen bonds to unsaturated organic compounds, like alkenes and alkynes, is what hydrosilylation is all about. Diverse industries use this process to produce silicon-containing products.
2). What is the significance of regio-divergent hydrosilylation?
Regio-divergent hydrosilylation allows for the preferential creation of distinct products from identical initial substances, thereby improving the adaptability and effectiveness of synthetic pathways in organic chemistry.
3). What are the properties of nickel that make it an effective catalyst for regio-divergent hydrosilylation?
Nickel is an economical and easily accessible metal with distinctive electrical characteristics that improve reaction efficiency and selectivity. Therefore, it is a highly suitable option for catalytic hydrosilylation.
4). What is the method of synthesizing α-(fluoroalkyl)styrenes?
α-(fluoroalkyl)styrenes are commonly produced using conventional organic reactions that involve fluoroalkyl groups and styrene derivatives. It is crucial to exercise precise control to preserve the integrity of the fluoroalkyl group during the synthesis process.
5). What are the main purposes of this research?
To make more advanced materials, nickel-catalyzed regio-divergent hydrosilylation of α-(fluoroalkyl)styrenes are mostly used in materials science to create new materials, pharmaceuticals to create useful intermediates, and agrochemicals to make agricultural compounds more active and stable.
For more chemistry blogs, visit chemistry Master