Table of Contents
Overview of Oxygen Reduction Reaction:
The oxygen reduction reaction (ORR) is a fundamental mechanism in electrochemical energy conversion devices such as fuel cells and metal-air batteries. Platinum, a catalyst renowned for its remarkable catalytic characteristics, is the central focus of this essential response. Nevertheless, the effectiveness of platinum in catalyzing the oxygen reduction reaction (ORR) is not without difficulties, which has led researchers to investigate novel methods to enhance its performance. An intriguing option involves the in-situ adjustment of platinum’s 5d valence states, a technique that has the potential to improve the efficiency of oxygen reduction reactions (ORR) by enabling a more efficient four-electron reduction route.
Comprehending the Oxygen Reduction Reaction (ORR):
The oxygen reduction reaction is a crucial process in energy conversion devices, in which oxygen is chemically transformed to produce water or hydrogen peroxide. Oxygen reduction reaction (ORR) can occur through two main pathways: a four-electron channel that directly converts oxygen into water, and a two-electron mechanism that converts oxygen into hydrogen peroxide. The four-electron pathway is highly advantageous for energy applications because of its superior efficiency and less generation of undesirable by-products. Schematic illustration.
The Significance of Platinum in Oxygen Reduction Reaction (ORR):
Platinum is widely acknowledged as a top-tier catalyst for the oxygen reduction reaction (ORR) because of its exceptional catalytic activity and stability. Although platinum surfaces possess advantageous characteristics, the process of ORR (oxygen reduction reaction) is frequently impeded by several obstacles, such as slow reaction rates and excessive overpotentials. These constraints have prompted extensive research endeavors to improve the catalytic effectiveness of platinum, with a specific emphasis on refining its electrical configuration and surface characteristics.
The significance of valence states:
Valence states pertain to the oxidation state of atoms within a molecule and have a significant impact on the catalytic behavior of a substance. The valence state of the active site in catalytic processes has a significant impact on the adsorption and activation of reactants, which in turn affects the total reaction rate. Comprehending and managing valence states is crucial for maximizing catalyst efficiency.
The valence states of platinum in 5D orbitals are examined:
Platinum’s 5D orbitals, specifically the 5D valence states, primarily shape its catalytic characteristics and significantly influence the interaction between platinum and oxygen species. Being able to adjust these valence states in real-time while the ORR process is happening can result in significant enhancements in catalytic efficiency. This has become a central focus of current electrocatalysis research.
In-Situ Tuning: Definition and Methods
Dynamically modifying a catalyst’s electrical and structural characteristics during its use is known as in-situ tuning. This method enables the catalyst’s performance to be optimized during the reaction, providing a dynamic approach to improving catalytic efficiency. We can adjust and observe the valence states of platinum in its original location using methods like electrochemical and spectroscopic techniques. Morphology and phase characterizations.
The mechanism of the Four-Electron Oxygen Reduction Reaction (ORR) occurs on platinum:
The four-electron pathway of the oxygen reduction reaction (ORR) on platinum involves the direct conversion of oxygen into water, which is a more efficient process than the two-electron pathway. The 5D valence states of platinum play a crucial role in enabling this process by affecting the adsorption, activation, and reduction of oxygen molecules. Understanding this mechanism in depth is crucial for devising approaches to improve the performance of the oxygen reduction reaction (ORR) by adjusting the valence state.
Methods for tuning in situ through experimentation:
Researchers utilize a variety of experimental approaches to adjust and examine the 5D valence states of platinum. These techniques encompass electrochemical methods such as cyclic voltammetry and chronoamperometry, as well as spectroscopic approaches like X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). These techniques offer an in-depth analysis of the electrical and structural modifications taking place in the catalyst during the oxygen reduction reaction (ORR).
Electrochemical techniques:
Electrochemical techniques are essential for investigating the oxygen reduction reaction (ORR) and adjusting the oxidation states of platinum. We employ cyclic voltammetry to examine the electrochemical characteristics of the catalyst and identify reaction intermediates, while chronoamperometry provides valuable insights into the catalyst’s durability and effectiveness during continuous operation. These strategies are critical for understanding the valence-state adjustment dynamics during ORR.
Spectroscopic techniques:
Spectroscopic techniques provide comprehensive information about the electrical configuration and oxidation states of platinum. X-ray absorption spectroscopy (XAS) helps us understand the link between valence states and catalytic activity by showing us the oxidation states and close surroundings of platinum atoms. We employ X-ray photoelectron spectroscopy (XPS) to examine the surface chemistry and electronic states of platinum, which provides vital information on the impact of adjusting the valence state on the performance of the oxygen reduction reaction (ORR). Electrochemical performance.
Adjusting the valence state affects the performance of the oxygen reduction reaction (ORR):
Modulating the 5D valence states of platinum can result in substantial improvements in oxygen reduction reaction (ORR) efficiency. Studies have demonstrated that optimizing the valence states improves oxygen molecule adsorption and activation, reduces activation energy, and increases catalytic activity. Comparative studies demonstrate improvements in important metrics related to the oxygen reduction reaction (ORR), such as current density, onset potential, and overall efficiency, achieved through successful valence state adjustment. Local structure and electronic structure characterizations.
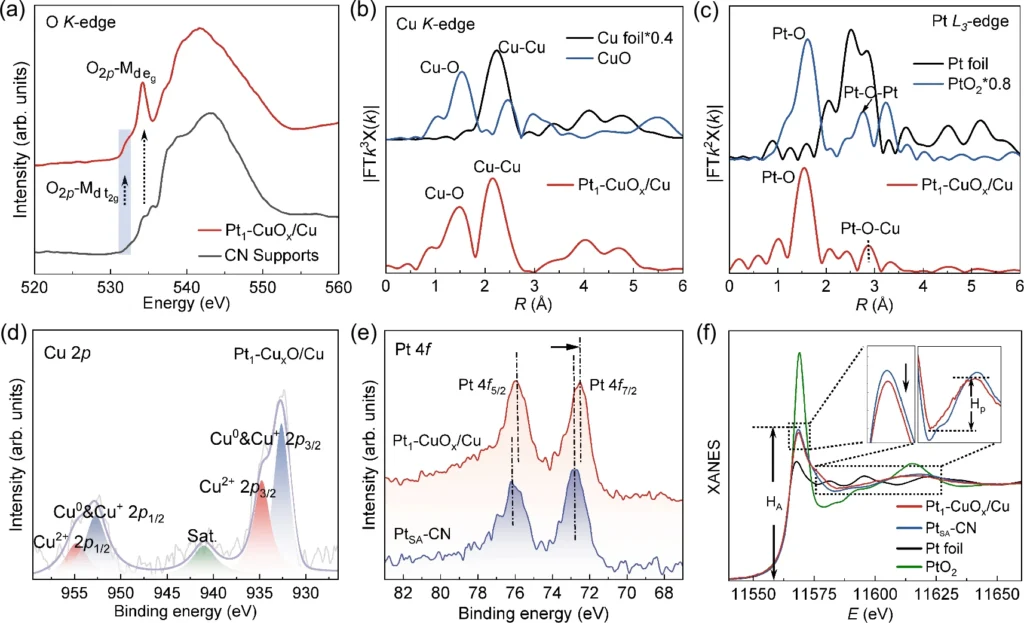
Understanding Valence State Tuning: Exploring Theoretical Approaches
It’s important to use theoretical methods like density functional theory (DFT) and computational modeling to understand and predict what will happen when the valence state in the oxygen reduction reaction (ORR) is changed. These methods give useful details about the electrical structure, adsorption energies, and reaction paths of oxygen species on platinum surfaces. This can help scientists make catalysts that work better. Researchers can gain a thorough grasp of the mechanisms that control valence state tuning by integrating experimental and theoretical methods.
Difficulties with -Situ Tuning:
Despite the potential for in-situ adjustment in platinum valence states, various issues require attention. Technical challenges include maintaining stable tuning conditions, guaranteeing catalyst longevity, and achieving consistent performance in real-world working situations. Furthermore, changing the valence state is very complicated and requires complex experimental setups and careful control of response parameters, which makes things very hard for researchers.
The research includes an analysis of specific instances and notable findings:
Multiple studies have emphasized the advantages of adjusting the valence states of platinum in its original location for oxygen reduction reactions (ORR). Notable research findings demonstrate that optimizing the tuning of valence states has led to significant advancements in catalytic efficiency and stability. Case studies include comprehensive descriptions of this research’s experimental methods, strategies, and results, providing a significant understanding of the practical uses and advantages of in-situ tuning.
There are other applications beyond the Oxygen Reduction Reaction (ORR):
While valence state tuning is the primary application of ORR, its potential extends beyond oxygen reduction. For instance, various electrochemical reactions like the hydrogen evolution reaction (HER) and the methanol oxidation reaction (MOR) can benefit from modifying the valence states of platinum in its original location. By applying the principles of valence state tuning to these processes, scientists can create electrocatalysts that are more effective and adaptable for various purposes. In situ XAFS measurements.
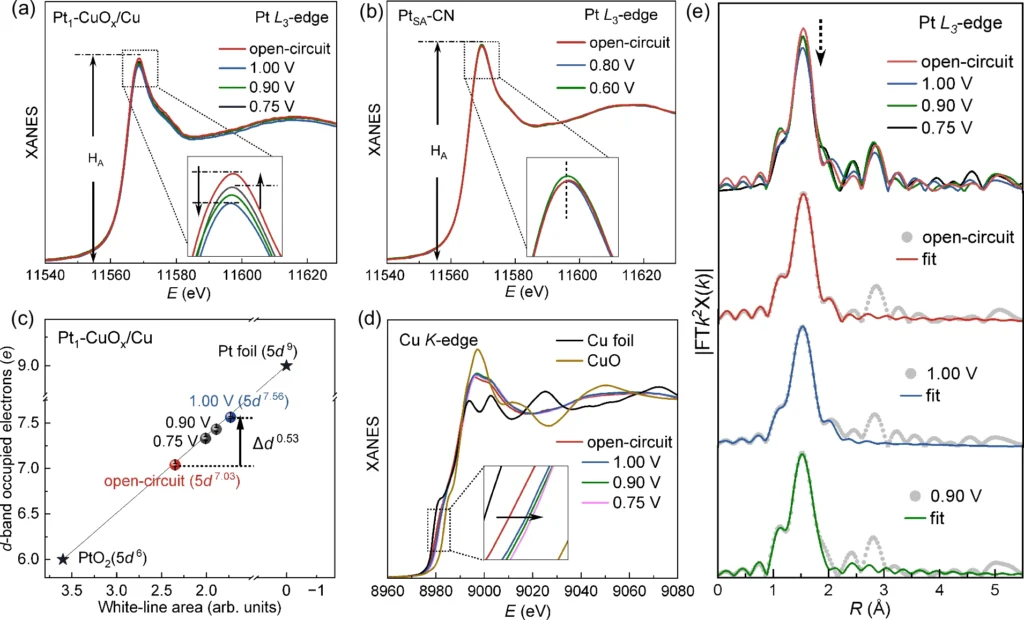
There are prospects for advancements in Oxygen Reduction Reaction (ORR) catalysis:
Oxygen reduction reaction (ORR) catalysis is undergoing tremendous advancements as researchers continue to focus on the development of catalysts that are both more efficient and long-lasting. Potential future avenues of research include investigating alternate materials, such as non-platinum-group metals and metal alloys, as well as employing sophisticated tuning techniques that provide enhanced manipulation of valence states. In addition, scientists are researching to acquire a more profound comprehension of the fundamental principles that drive the oxygen reduction reaction (ORR). Scientists will then use this knowledge to guide the development of catalysts for the next generation.
In conclusion:
A possible way to make the four-electron oxygen reduction process work better is to change the platinum 5D valence states back to where they were before. Researchers can make substantial improvements in the performance of oxygen reduction reactions (ORR) by optimizing platinum’s electrical characteristics, thereby facilitating the development of more efficient and sustainable energy conversion systems. Continued research on this subject will lead to the advancement of creative tuning approaches and a more profound understanding of the underlying mechanics, which will further drive electrocatalysis advancements.
Frequently Asked Questions:
1). What is the significance of the 5D valence state in platinum for the oxygen reduction reaction (ORR)?
The 5D valence state of platinum is a key part of its catalytic properties; it affects how oxygen molecules bind to and become active in the oxygen reduction reaction. Optimizing these valence states can enhance the efficiency and performance of the oxygen reduction reaction (ORR).
2). What is the impact of in-situ tweaking on improving the oxygen reduction reaction’s (ORR) efficiency?
In-situ tuning allows for immediate modification of platinum’s electrical and structural characteristics, boosting its interaction with oxygen species and improving the kinetics and efficiency of the oxygen reduction reaction (ORR). By employing this dynamic technique, researchers can modify the catalyst parameters in real-time during the reaction, resulting in enhanced performance.
3). What are the typical methods used for in-situ tuning?
Electrochemical techniques like cyclic voltammetry and chronoamperometry are often used for tuning in situ, as well as spectroscopic techniques like X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). These methods offer a comprehensive understanding of the electrical and structural modifications taking place in the catalyst during the oxygen reduction reaction (ORR).
4). What challenges are associated with the in-situ adjustment of platinum valence states?
In-situ tuning of platinum valence states is hard because you have to keep the tuning conditions stable, make sure the catalyst lasts a long time, and get consistent performance in real-world settings. Correctly adjusting the valence state necessitates intricate experimental arrangements and meticulous management of reaction conditions.
5). What are the potential avenues for study in this field?
Some possible areas for future research on this subject are looking into different catalyst materials, improving tuning methods to give us more precise control over valence states, and learning more about the basic rules that govern the oxygen reduction reaction (ORR). These endeavors will facilitate the advancement of more effective and environmentally friendly electrocatalysts for a diverse array of uses.
For more chemistry blogs, visit chemistry Master