Table of Contents
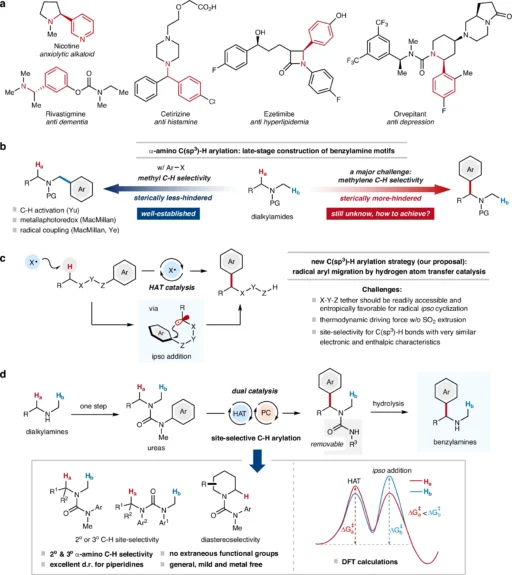
Overview of α-C(sp3)–H Arylation:
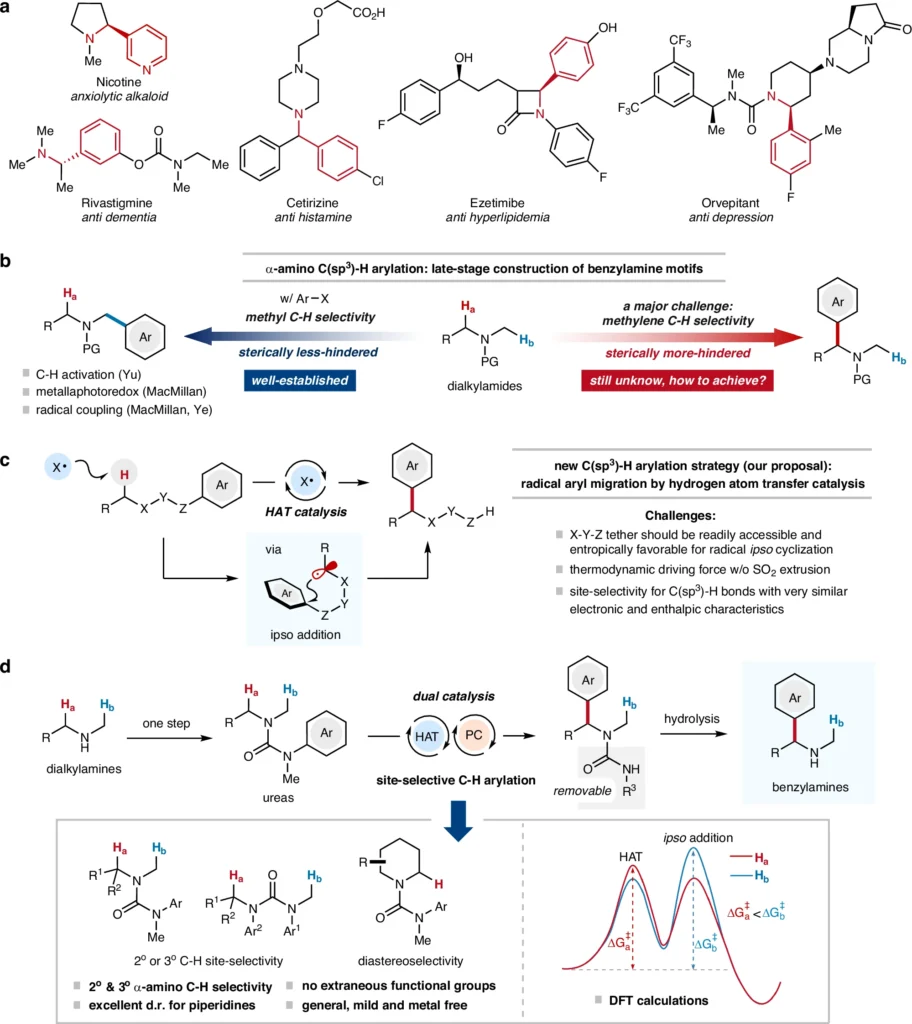
α-C(sp3)–H Arylation, which involves incorporating an aryl group into a molecule, is a crucial process in organic chemistry, especially in the creation of intricate compounds such as medicines and agrochemicals. Among the various techniques for introducing α-C(sp3)–H aryl groups, the α-C(sp3)–H arylation method has attracted considerable interest because it allows for the modification of molecules without the need for pre-functionalized starting materials. Even so, getting site-selectivity is still a big problem, especially when it comes to the α-C(sp3)-H arylation of alkylamines because these bonds don’t react easily and it’s hard to control regioselectivity.
Dialkylamines are highly useful compounds in the field of organic synthesis, providing a wide range of possible uses. Nevertheless, the α-C(sp3)-H bonds in these compounds are often unreactive, which poses a challenge for achieving selective functionalization. Site-selective arylation methods can greatly improve the creation of complex organic molecules, opening up new ways to make compounds with precise changes to their structures. Site-selective α-C(sp3)–H arylation for the construction of benzylamines.
Principles of Hydrogen-Atom Transfer (HAT) Catalysis:
Hydrogen Atom Transfer (HAT) is a key mechanism in organic chemistry that entails the transfer of a hydrogen atom from one molecule to another. This process can produce radicals, which are extremely reactive intermediates capable of engaging in a broad spectrum of chemical reactions. In the context of α-C(sp3)-H arylation, hydrogen atom transfer (HAT) catalysis is highly advantageous as it enables the creation of carbon-centered radicals, which can subsequently undergo further chemical reactions.
HAT catalysis commonly employs a catalyst that removes a hydrogen atom from the target substrate, resulting in the formation of a radical intermediate. Subsequently, this intermediate can undergo a reaction with an arylating agent, leading to the synthesis of the intended arylated product. The selection of catalysts and reaction conditions plays a critical role in defining the effectiveness and specificity of the HAT process.
The Concept of Radical α-C(sp3)–H Arylation:
Radical arylation is a highly effective technique in organic synthesis that allows for the creation of carbon-carbon bonds gently. Radical arylation offers a more gentle and eco-friendly alternative to classic arylation procedures, which typically involve the use of harsh chemicals and conditions. This technique entails the creation of a radical intermediate, which subsequently undergoes a reaction with an aryl source to produce the arylated product.
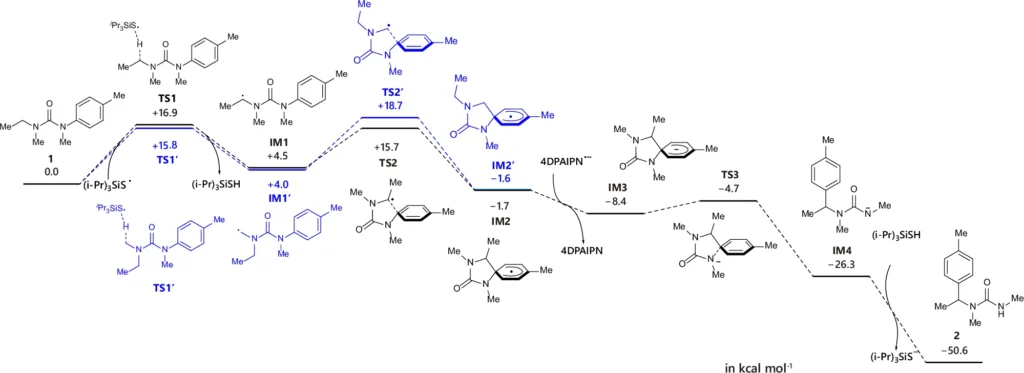
Utilizing radical chemistry in arylation provides increased versatility in selecting substrates and reaction conditions. This method is especially valuable in the process of introducing aryl groups into α-C(sp3)–H bonds, where conventional techniques may face challenges in achieving the appropriate level of selectivity and efficiency. Theoretical calculation of reaction mechanism (ethyl pathway, in black) with comparison with methyl pathway (in blue).
Proposed mechanism of α-C(sp3)–H.
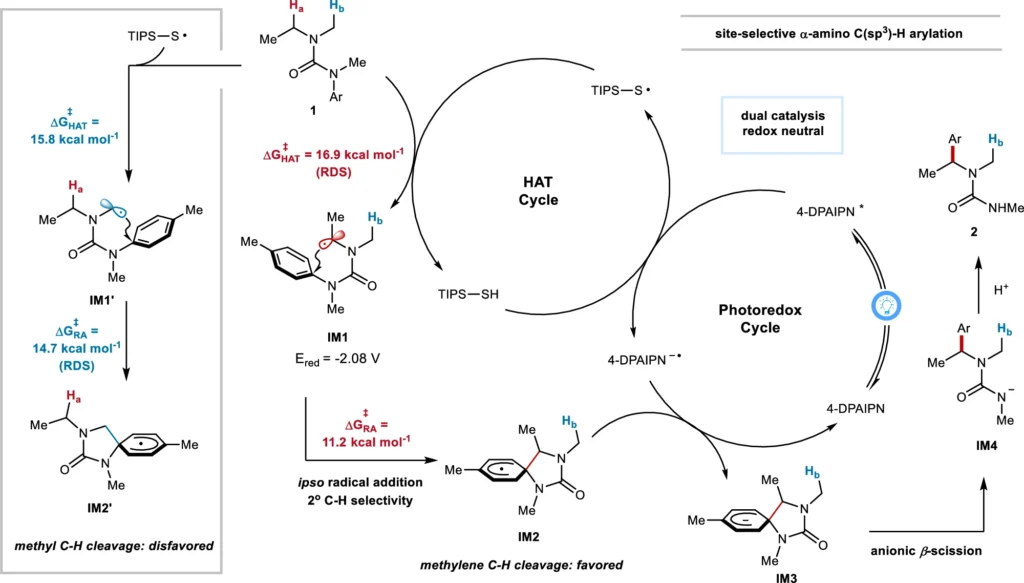
Advancement of Site-Selective α-C(sp3)-H Arylation:
The search for a method to selectively target certain carbon-hydrogen α-C(sp3)–H bonds for arylation has prompted the investigation of many strategies, each with its strengths and weaknesses. In the past, it has been difficult to achieve selectivity in α-C(sp3)–H functionalization since numerous C-H bonds within a molecule have identical reactivity. Recent developments in HAT catalysis have created new opportunities for targeted arylation, especially when applied to dialkylamines.
The importance of α-C(sp3)-H arylation lies in its ability to alter the fundamental structure of molecules, providing novel prospects for the creation of intricate, functionalized compounds. This strategy is very valuable in altering medications and other biologically active substances, as it typically necessitates precise structural alterations.
Catalysis-Enabled Radical α-C(sp3)–H Arylation: A Significant Advancement
The incorporation of HAT catalysis into radical arylation marks a significant advancement in organic synthesis. Chemists can perform site-selective arylation of α-C(sp3)-H bonds in alkylamines by utilizing the power of HAT to produce carbon-centered radicals. This approach provides numerous benefits compared to conventional arylation methods, such as enhanced selectivity, gentler reaction conditions, and the capability to modify substrates that were previously unresponsive.
The process of radical arylation through hydrogen atom transfer (HAT) entails the removal of a hydrogen atom from the α-C(sp3)-H bond by a catalyst, resulting in the formation of a carbon-centered radical. Subsequently, this radical can undergo aryl migration, leading to the creation of the arylated product. The reaction’s outcome is significantly influenced by the catalyst, aryl source, and reaction conditions selected.
Utilization of Radical Arylation in Dialkylamines:
The structural features and the presence of α-C(sp3)-H bonds make alkylamines very suitable for radical arylation. The successful introduction of aryl groups into these molecules creates new opportunities for synthesizing a diverse array of functionalized amines. These amines are important intermediates in the production of medicinal and agrochemical chemicals.
Researchers have investigated radical arylation processes using different dialkylamine substrates, with varying degrees of success. Factors such as the substituents on the amine, the chosen catalyst, and the reaction conditions can influence the efficiency and selectivity of the arylation process. Scope of acyclic amine-derived ureas in site-selective C(sp3)–H arylation.
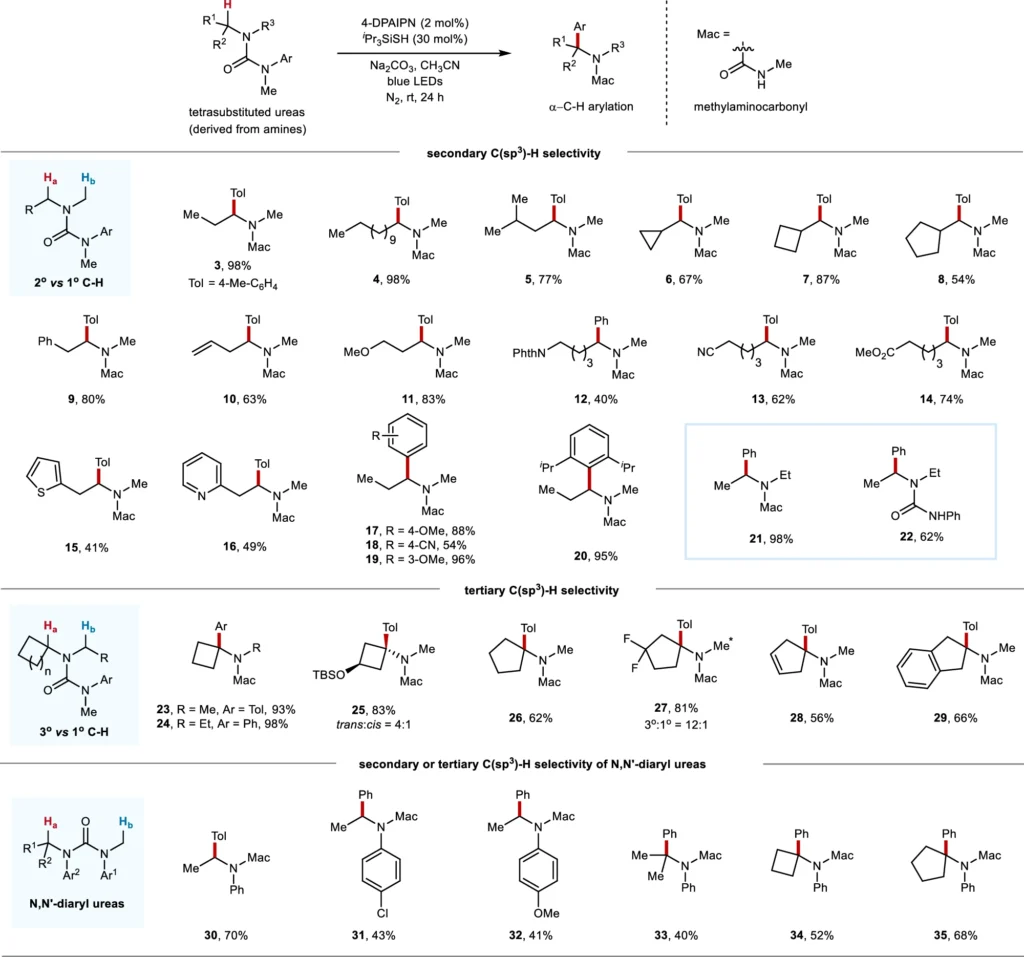
Explanation of the Radical Aryl Migration Mechanism:
The process of radical aryl migration is an intricate mechanism that encompasses multiple crucial steps. The first stage entails the production of a carbon-centered radical using hydrogen atom transfer (HAT) catalysis. The radical subsequently reacts with an aryl compound, resulting in the creation of an arylated intermediate. The last step entails translocating the aryl group to the intended location inside the molecule.
Understanding the pivotal intermediates and transition states in this process is critical for fine-tuning the reaction conditions and improving the overall efficacy of the arylation process. Various factors, including the stability of the radical intermediate, the characteristics of the aryl source, and the conditions of the reaction environment, determine the outcome of the reaction.
Methods and Procedures for Conducting Experiments:
Accomplishing radical arylation effectively necessitates a meticulous evaluation of the experimental circumstances. Crucial considerations include catalyst selection, aryl source characteristics, and reaction temperature. Typical catalysts utilized for HAT-enabled radical arylation consist of metal complexes and organic compounds that may remove hydrogen atoms from α-C(sp3)–H bonds.
Optimal reaction conditions are necessary to achieve efficient hydrogen atom transfer and subsequent arylation. We use commonly employed analytical techniques like NMR spectroscopy, mass spectrometry, and chromatography to monitor the progress of the reaction and analyze the resulting products.
Obstacles and Constraints:
Despite the numerous benefits of HAT-enabled radical arylation, it still faces various obstacles that require attention. A significant challenge is achieving precise control over regioselectivity, especially in intricate structures containing numerous C(sp3)-H bonds. In addition, the stability of the radical intermediates might be a constraining factor, as extremely reactive radicals may result in side reactions or breakdowns.
Another obstacle is the ability to scale up the reaction, particularly in industrial environments where significant amounts of arylated compounds are required. Methods to address these difficulties include creating catalysts with greater selectivity, fine-tuning reaction conditions, and employing protecting groups to regulate reactivity.
Case Studies: Examples of Successful α-C(sp3)–H Arylation
Multiple instances of effective α-C(sp3)-H arylation in dialkyl amines have been documented in the literature. These investigations emphasize the capability of HAT-enabled radical arylation for creating intricate chemical compounds. Notable examples involve the introduction of aryl groups into amines, a common practice in pharmaceutical production. In this context, precise targeting and achieving a high percentage of desired products are of utmost importance.
These case studies demonstrate the adaptability of the approach and its potential for use in various chemical syntheses. The evaluations of this research often rely on the product yield, purity, and level of site-selectivity attained. Protecting group removal experiments and mechanistic studies.
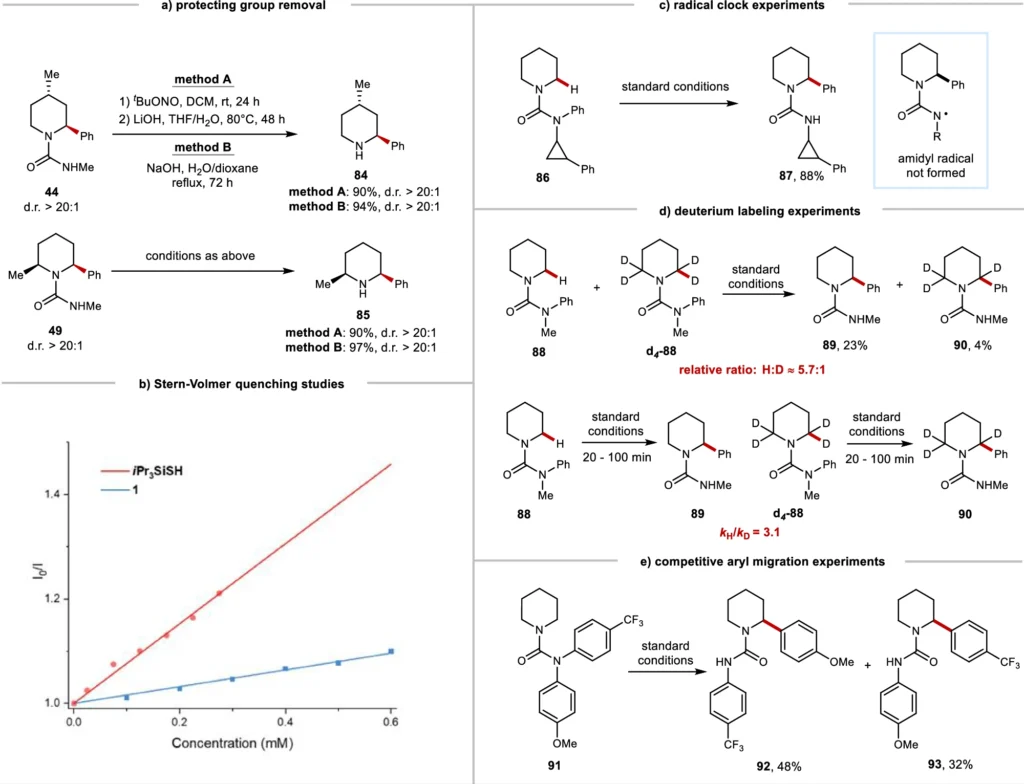
Effects on the Field of Synthetic Organic Chemistry:
The advancement of site-selective α-C(sp3)-H arylation has the potential to have a substantial influence on the domain of synthetic organic chemistry. This technique provides a novel strategy for modifying complicated molecules, enabling the accurate alteration of chemical structures. The capacity to specifically introduce aryl groups into α-C(sp3)–H bonds in dialkyl amines presents novel opportunities for producing medicines, agrochemicals, and other useful molecules.
HAT-enabled radical arylation has many benefits over traditional arylation methods, including better selectivity, gentler reaction conditions, and the ability to change substrates that weren’t reachable before. This is an appealing choice for both academic and industry chemists because of its advantages.
Prospects for further advancements in α-C(sp3)-H arylation:
The prospects for advancing site-selective α-C(sp3)-H arylation are encouraging, offering numerous opportunities for further investigation and progress. We expect advancements in catalyst design, reaction conditions, and mechanistic understanding to enhance the efficiency and selectivity of the process. Furthermore, using this technique on a wider range of substrates and functional groups has the potential to improve its usefulness in organic synthesis.
Ongoing studies in radical chemistry and hydrogen atom transfer are anticipated to enhance the progress of α-C(sp3)-H arylation techniques. In the future, this method has the potential to become a commonly used technique for creating intricate organic compounds.
Environmental and economic factors are to be taken into account:
HAT-enabled radical arylation has the important benefit of being an environmentally friendly and sustainable method for chemical synthesis. This method is more environmentally friendly than standard arylation techniques due to its utilization of mild reaction conditions and the avoidance of harsh chemicals. Moreover, the capacity to modify unreactive α-C(sp3)–H bonds without requiring pre-modified initial substances can minimize waste and enhance the overall efficiency of the synthesis.
The reaction’s scalability and cost-efficiency are critical economic factors to consider. Improving catalysts and reaction conditions could increase the economic feasibility of this approach, making it a desirable choice for large-scale chemical manufacturing.
In conclusion:
The selective introduction of an aryl group to a specific carbon-hydrogen bond α-C(sp3)–H in dialkylamines using a catalytic process that involves the transfer of a hydrogen atom and enables the migration of a radical aryl group is notable progress in the field of organic synthesis. This method offers a unique approach to altering complex molecules, potentially finding applications in diverse industries such as pharmaceuticals and agrochemicals. This technology can potentially revolutionize chemists’ approach to synthesizing complex organic compounds, providing novel prospects for innovation and exploration.
FAQs:
1. Why is α-C(sp3)-H arylation significant in organic chemistry?
α-C(sp3)-H arylation is essential as it enables the specific alteration of carbon-hydrogen bonds, facilitating the creation of intricate compounds with great accuracy. This α-C(sp3)–H is especially beneficial in the pharmaceutical sector, where accurate structural alterations may result in the creation of novel medications.
2. What is the mechanism of hydrogen atom transfer catalysis?
The process of Hydrogen Atom Transfer (HAT) catalysis involves the transfer of a hydrogen atom from one molecule to another, leading to the creation of a radical intermediate capable of participating in subsequent chemical reactions. A catalyst facilitates this process by removing the hydrogen atom, creating a highly reactive radical capable of undergoing several transformations, including arylation.
3. What are the primary obstacles to achieving site-selective arylation?
The main obstacles in site-selective arylation include regulating the reaction’s regioselectivity, handling radical intermediate reactivity, and adjusting reaction conditions to achieve high yields and selectivity. Moreover, the process of increasing the scale of the reaction for industrial purposes can present difficulties.
4. Is this method applicable to other types of amines?
Indeed, while this article primarily focuses on dialkylamines, other categories of amines could potentially benefit from the fundamental concepts of HAT-enabled radical arylation. However, the precise responsiveness and specificity will depend on the characteristics of the amine and the conditions under which the reaction takes place.
5. What are the ecological advantages of this α-C(sp3)–H arylation technique?
The environmental advantages of HAT-enabled radical arylation include the use of gentler reaction conditions, waste minimization by circumventing pre-functionalized starting materials, and the possibility of a more sustainable synthesis process. These elements contribute to a more environmentally friendly approach to chemical synthesis.
For more chemistry blogs, visit chemistry Master