Table of Contents
Opening statement of Asymmetric Annulation:
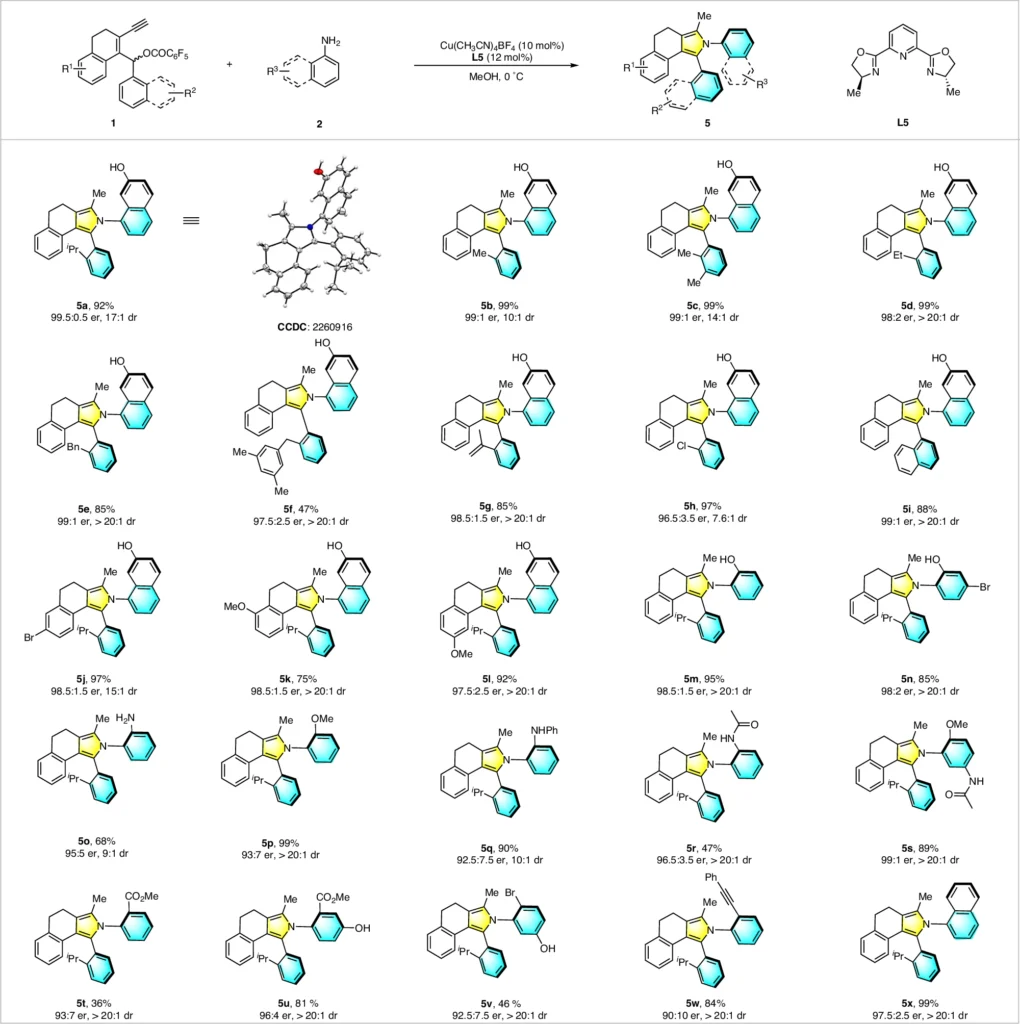
Organic chemistry offers a captivating realm where asymmetric annulation has become a potent technique for creating intricate structures with specific stereochemistry. Researchers are very interested in how copper can be a catalyst to help yne-allylic esters and amines react unevenly. This reaction produces axially chiral aryl pyrroles in a precise and sophisticated manner. These compounds possess structural fascination and significant potential in many disciplines, such as pharmacology and material research.
Therefore, what makes this reaction highly significant? What are the specific reasons that make copper the preferred catalyst? Now, let us delve into and examine the complexities of this captivating chemical metamorphosis.
Comprehending Yne-Allylic Esters:
Before delving into the details of the reaction, it is critical to understand the principal individuals involved. In their molecular structure, yne-allylic esters are chemical compounds that contain both an alkyne (yne) and an allylic ester group. These molecules are extremely adaptable compounds used in organic synthesis, renowned for their capacity to engage in a wide range of reactions, such as cyclizations and cross-couplings. These esters are very reactive due to the presence of both alkyne and ester functions, which allows for intricate chemical changes.
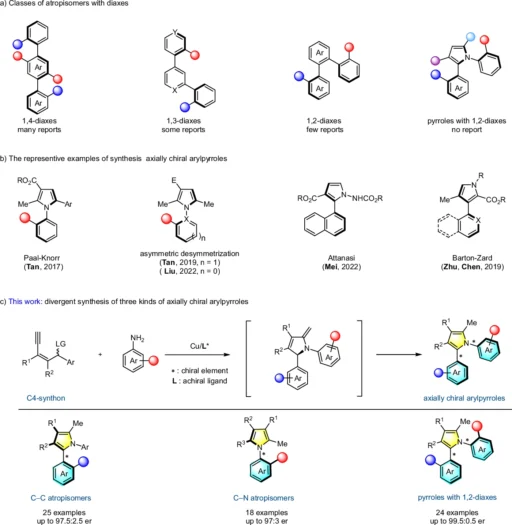
In the past, organic synthesis has utilized yne-allylic esters to create a diverse range of heterocyclic molecules. Because of their ability to undergo annulation reactions, they have become highly useful components in synthesizing natural goods and medicines. Development of axially chiral arylpyrroles.
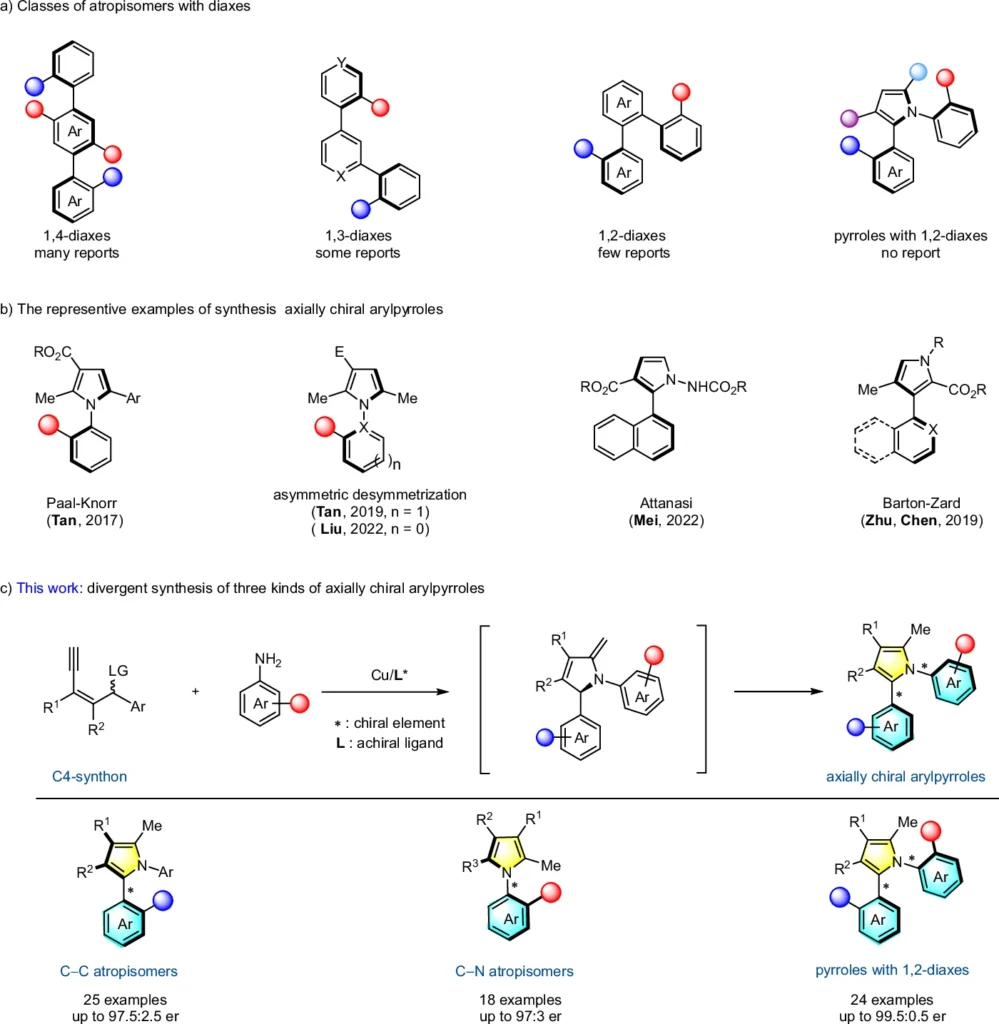
The Concept of Asymmetric Annulation:
Asymmetric annulation is a cyclization reaction that results in the formation of a new ring and one or more stereocenters. This procedure is crucial in the field of stereoselective synthesis, where the objective is to generate molecules with a precise three-dimensional configuration. The term “asymmetric” pertains to the selective production of one enantiomer over the other, a critical factor for the biological efficacy of numerous substances.
In the context of yne-allylic esters, asymmetric annulation means making a ring structure, which leads to the production of aryl pyrroles that are chiral along their length. The ability to manipulate asymmetric annulation stereochemistry during this procedure is what makes this reaction so significant.
Copper-Catalyzed Reactions of asymmetric annulation :
Catalysis has historically widely used copper. The versatility of its capacity to facilitate a diverse array of chemical reactions, combined with its cost-effectiveness and eco-friendliness, renders it a highly appealing option for catalysis. Copper is an important part of the yne-allylic ester annulation process because it speeds up the reaction and keeps the stereoselectivity high.
What is the rationale for choosing copper? People recognize copper catalysts for their ability to stabilize transition states and encourage the formation of carbon-carbon and carbon-heteroatom bonds. These catalysts are well-suited for facilitating intricate annulation processes, which require the controlled formation of numerous bonds.
Furthermore, copper’s versatility includes its ability to cooperate with chiral ligands, which are crucial for producing asymmetry in the reaction. By working with these ligands, copper speeds up the reaction that makes a certain enantiomer, ensuring the desired stereochemical outcome.
Amines are reactants:
Amines function as the nucleophilic counterparts that undergo a reaction with the yne-allylic esters. The amine selection is critical, as it can have a significant impact on the reaction’s efficiency and selectivity. Different amines could change the electrical and steric properties of the intermediates, which could change the stereochemical outcome of the annulation process.
These reactions frequently employ amines, both primary and secondary. Various circumstances, including the existence of electron-donating or electron-withdrawing groups, affect the reactivity and selectivity of these amines. Chemists can fine-tune these features in the reaction to achieve the best yield and selectivity of axially chiral aryl pyrroles.
Mechanism of Copper-Catalysed Asymmetric Annulation:
To will Asymmetric Annulation the reaction mechanism to comprehend how copper catalysis facilitates the creation of axially chiral aryl pyrroles.
Activation of the Yne-Allylic Ester: The coordination of the copper catalyst activates the Yne-Allylic Ester, enabling nucleophilic assault. This activation is essential because it reduces the energy barrier for the following reaction stages.
Nucleophilic Attack by the Amine: A nucleophilic attack is made on the activated ester by the amine. This creates an intermediate that is ready to go through cyclization.
Cyclization and Formation of Axially Chiral Center: The copper catalyst aids the intermediate in a cyclization reaction that forms an axially chiral center. In this stage, the cooperative action of the copper catalyst and chiral ligand imparts the axial chirality, ensuring that the freshly produced ring assumes a precise three-dimensional configuration.
Final Product Formation: The axially chiral aryl pyrrole is freed from the catalyst in the last step, which ends the reaction.
The copper catalyst not only speeds up the reaction, but it also has a big effect on the stereochemistry, which makes it possible to make products with a lot of enantioenrichment. Gram-scale synthesis, synthetic transformations, and mechanistic investigations of asymmetric annulation .
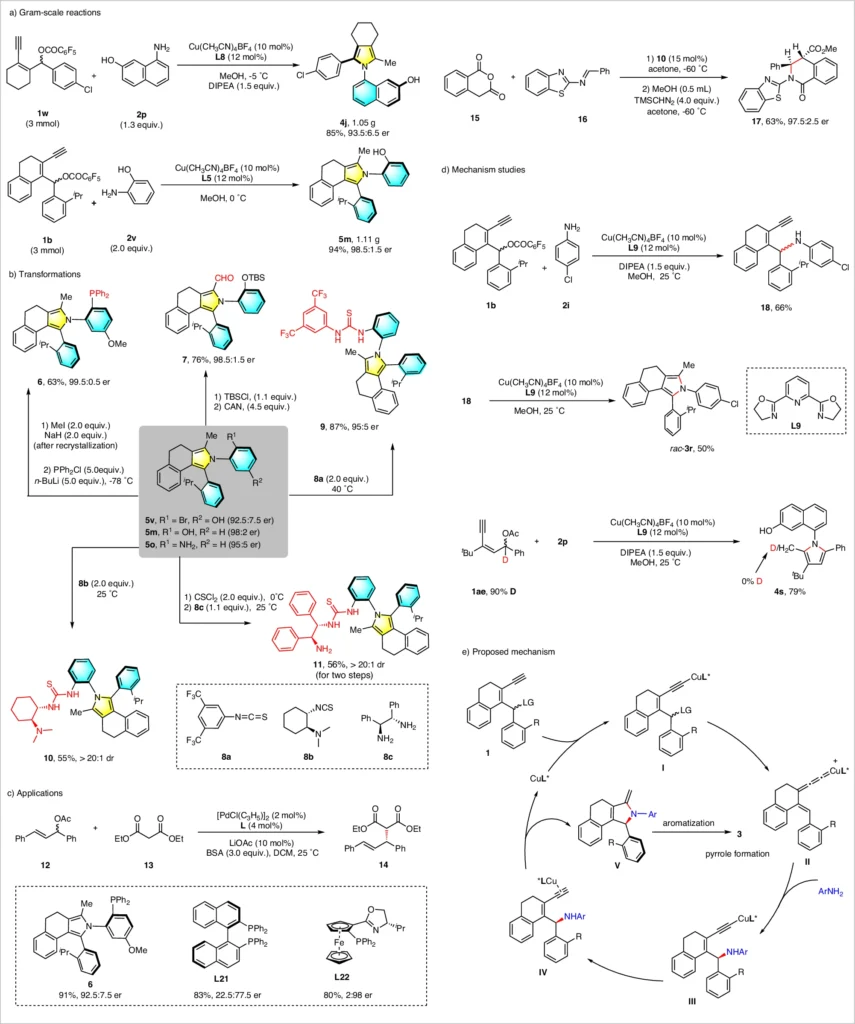
Stereoselectivity in the Reaction:
Attaining a high level of stereoselectivity poses a significant obstacle in the field of asymmetric synthesis. Numerous factors influence the stereoselectivity of the copper-catalyzed annulation of yne-allylic esters.
Chiral Ligands: Chiral ligands play a crucial role in imparting chirality to the resulting molecule. The choice of ligand can have a significant impact on enantioselectivity, as different ligands offer varying degrees of control over the stereochemical result.
Reaction Conditions: Reaction conditions, such as temperature, solvent, and concentration, can all have an impact on a reaction’s stereoselectivity. Lower temperatures typically enhance higher enantioselectivity by decreasing the pace of competing, non-stereoselective processes.
Substrate Structure: The structure of both the yne-allylic ester and the amine in the substrate can influence the stereochemical result. If there are substituents on either the ester or the amine, they can create steric hindrance or electronic effects that change how the chiral center forms.
Experimental parameters:
It is crucial to optimize the reaction conditions to attain the highest feasible yield and selectivity. Typically, aprotic solvents like dichloromethane or tetrahydrofuran conduct these reactions without disrupting the catalytic cycle. Temperature regulation is essential, as lower temperatures often promote more enantioselectivity, but this comes at the expense of longer reaction durations.
Time is a crucial variable. Although longer reaction times may enhance yield, they can also result in side reactions or the breakdown of delicate intermediates. Hence, achieving the appropriate equilibrium is crucial for optimizing reactions effectively.
Illustrations of Effective Responses:
Recent research has shown several examples of copper-catalyzed asymmetric annulation processes that work well and create axially chiral aryl pyrroles. In a specific experiment, we paired a copper (I) catalyst with a chiral bisphosphine ligand to achieve high selectivity in the annulation process of a yne-allylic ester with a primary amine. This resulted in the synthesis of a highly enantioenriched aryl pyrrole with a fantastic yield.
In a different scenario, we used secondary amines and carefully adjusted the reaction conditions to obtain a final product with a significant level of axial chirality. These examples demonstrate the adaptability of the copper-catalyzed method and its capacity to produce intricate, asymmetrical molecules. Scope for the synthesis of 1,2-diaxes of axially chiral arylpyrrolesa.
Obstacles and Constraints:
Although the copper-catalyzed asymmetric annulation of yne-allylic esters has numerous benefits, it is not devoid of difficulties. The reaction’s susceptibility to moisture and air is an inherent problem, resulting in catalyst deactivation or unwanted side reactions. Reactions frequently take place in the presence of inert atmospheres like nitrogen or argon to address this issue.
Another obstacle is the possibility of conflicting secondary chemical reactions, such as ester hydrolysis or alkyne oligomerization. Prudent manipulation of the reaction conditions, such as employing protective groups or additives, can aid in alleviating these problems.
Comparative analysis:
Compared to previous approaches, the copper-catalyzed asymmetric annulation strategy for synthesizing axially chiral aryl pyrroles has numerous notable benefits. To begin, it makes it possible to directly create chiral centers with a high level of enantioselectivity, which could be hard to do with other catalytic systems. In addition, copper catalysts are typically more cost-effective and ecologically friendly in comparison to other transition metals such as palladium or rhodium.
Despite this, the copper-catalyzed asymmetric annulation method needs careful control over the reaction conditions and might not work with as many types of substrates as some other catalytic methods. Although there are certain limits, the copper-catalyzed asymmetric annulation technique remains a highly effective tool for synthetic chemists.
Utilization of Axially Chiral Aryl Pyrroles:
Axially chiral aryl pyrroles possess practical significance beyond being a mere subject of synthetic interest. They find concrete uses in various domains, notably in pharmaceuticals and material science. The pharmaceutical industry uses these compounds as crucial steps in producing medications with specific molecular structures, known as chiral centers. The arrangement of atoms in these structures can have a significant impact on the effectiveness and safety of the treatments.
Scientists studying materials are looking into axially chiral aryl pyrroles because they could be used to make new materials with unique optical or electrical properties. Due to their capacity to create stable, chiral structures, they are very desirable for use in chiral catalysis, molecular recognition, and the advancement of novel functional materials.
Prospects for the future:
In the future, there is a substantial possibility for the continued advancement of copper-catalyzed asymmetric annulation reactions. An intriguing field of study involves the investigation of novel chiral ligands that have the potential to enhance stereocontrol or facilitate reactions under less harsh conditions is an intriguing field of study. Furthermore, the process of expanding these reactions for use in industrial settings will be a crucial milestone in fully harnessing their capabilities in pharmaceutical production and other fields.
Applying this technology to different categories of esters or amines expands the range of chiral compounds possible. One of the main objectives is to create reaction conditions that are more ecologically friendly and sustainable, aligning with the overall effort to promote greener chemistry.
In conclusion:
The asymmetric annulation of yne-allylic esters with amines, which are catalyzed by copper, shows how advanced modern synthetic chemistry is at making complex, chiral compounds quickly and accurately. Chemists can use the unique properties of copper catalysis to make axially chiral aryl pyrroles that are very selective for one enantiomer over the other. This breakthrough offers exciting prospects in the fields of drug development, material science, and beyond.
This reaction not only demonstrates the efficacy of asymmetric catalysis but also emphasizes the significance of ongoing innovation in catalyst design and reaction optimization. We anticipate witnessing even more remarkable breakthroughs in the synthesis of chiral compounds as this field of research continues to advance.
Frequently Asked Questions:
1). What attributes of copper make it a favored catalyst for this reaction?
The preference for copper stems from its ability to stabilize transition states and encourage the formation of carbon-carbon and carbon-heteroatom bonds. Additionally, it is both economical and ecologically beneficial in comparison to alternative metal catalysts.
2). How does the amine selection influence the result?
The amine’s electronic and steric properties have a significant impact on the efficiency and selectivity of the reaction, which in turn affects the production of the chiral center and the total yield.
3). Is this strategy applicable to other categories of esters?
Indeed, by making suitable adjustments to the reaction conditions and catalyst system, it is possible to expand the scope of this approach to include various ester types, thereby increasing its versatility.
4). What are the possible industrial uses of axially chiral aryl pyrroles?
These compounds have significant use in the pharmaceutical industry for synthesizing medications with precise stereochemistry, as well as in material science for generating novel materials with distinct optical and electrical characteristics.
5). How does this method fare in terms of sustainability when compared to previous approaches?
The copper-catalyzed method is typically more environmentally friendly because copper has less toxicity and expense compared to metals such as palladium or rhodium. Nevertheless, additional research is required to enhance its green chemistry credentials.
For more chemistry blogs, visit chemistry Master