Table of Contents
Overview:
Chemistry is primarily concerned with comprehending the interactions and transformations of molecules. A complex phenomenon in the field involves the breaking of σ-bonds through unimolecular net heterolysis. This procedure entails the breaking of a chemical bond within a solitary molecule, resulting in the creation of two ions—a positively charged cation and a negatively charged anion. A detailed study of this process, especially when looking at symmetric and homopolar π-bonds, can teach us a lot about molecular stability, reactivity, and the underlying factors that control chemical processes.
We need to learn more about net heterolysis and other unimolecular reactions to better understand many chemical processes, from organic synthesis to industrial uses. In great detail, this article will go over the mechanics, thermodynamics, kinetics, and real-world effects of unimolecular net heterolysis in π-bonds that are symmetric and homopolar.
Analyzing Unimolecular Reactions:
To fully comprehend the concept of unimolecular net heterolysis, it is critical to first gain an understanding of its encompassing classification—unimolecular processes. Unimolecular reactions refer to chemical processes where a single molecule undergoes a transition without directly interacting with other molecules. These reactions are typically initiated by the molecule’s internal energy, which can come from thermal energy, light, or other types of energy input.
Unlike bimolecular reactions, which necessitate the collision of two reactant molecules, unimolecular reactions occur when a single molecule reaches an active state and subsequently undergoes a transformation. The activated state, commonly known as the transition state, is a temporary alteration of the molecule’s structure before it decomposes into products.
Isomerization and decomposition are common examples of unimolecular reactions. Isomerization changes the structure of a molecule without breaking any bonds. Decomposition breaks down a molecule into smaller pieces. An established illustration in the field of organic chemistry is the process of isomerization, specifically the conversion of cyclopropane to propene. When this change happens, the three-membered cyclopropane ring lets go of its stress, which makes the propene molecule more thermodynamically stable.
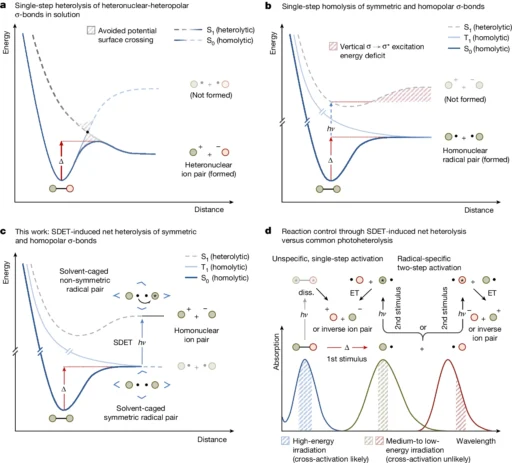
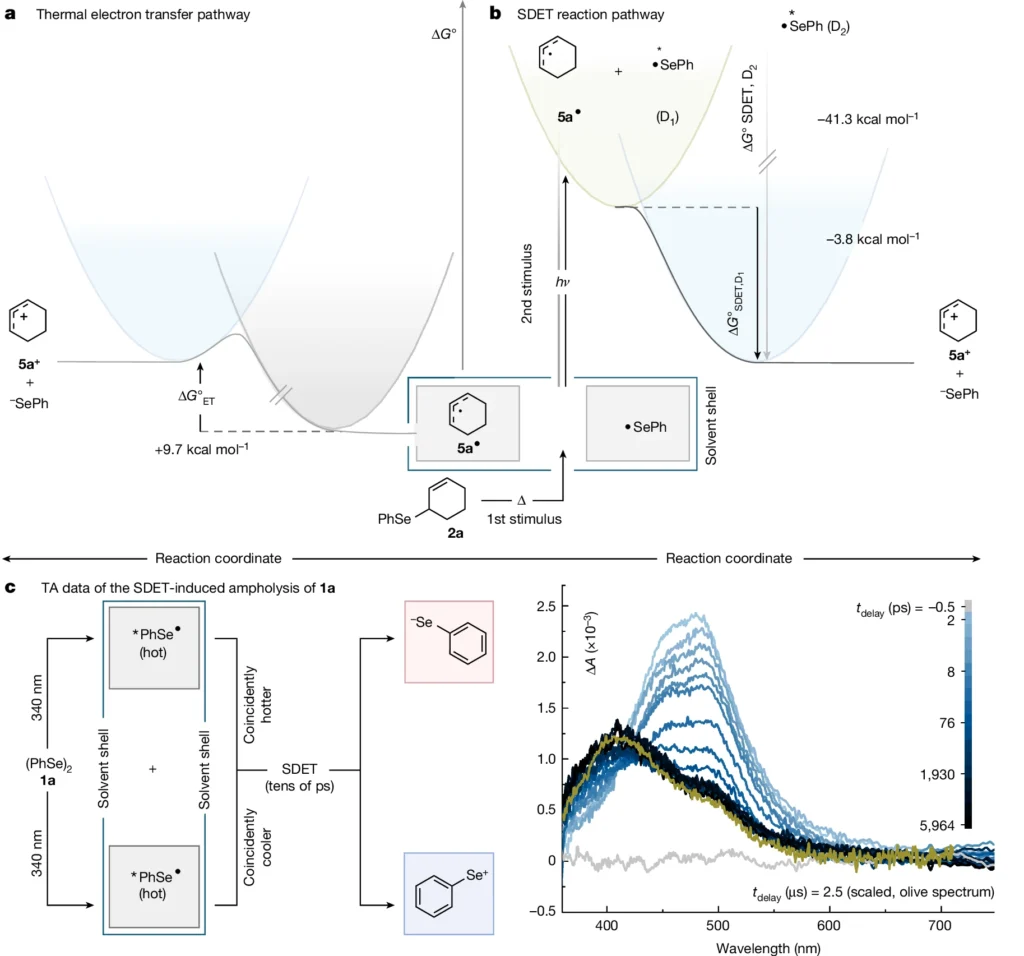
People commonly use the Lindemann-Hinshelwood method to explain unimolecular processes. This mechanism initially activates a molecule by colliding with other molecules or providing energy. In a subsequent phase, the activated molecule either decomposes or rearranges. The rate of these reactions is determined by the speed at which molecules reach the activated state and the likelihood that they will continue to form products once activated. Representation of potential energy surfaces for homo- and heteronuclear σ-bond fission and conceptualization of SDET-induced σ-bond net heterolysis.
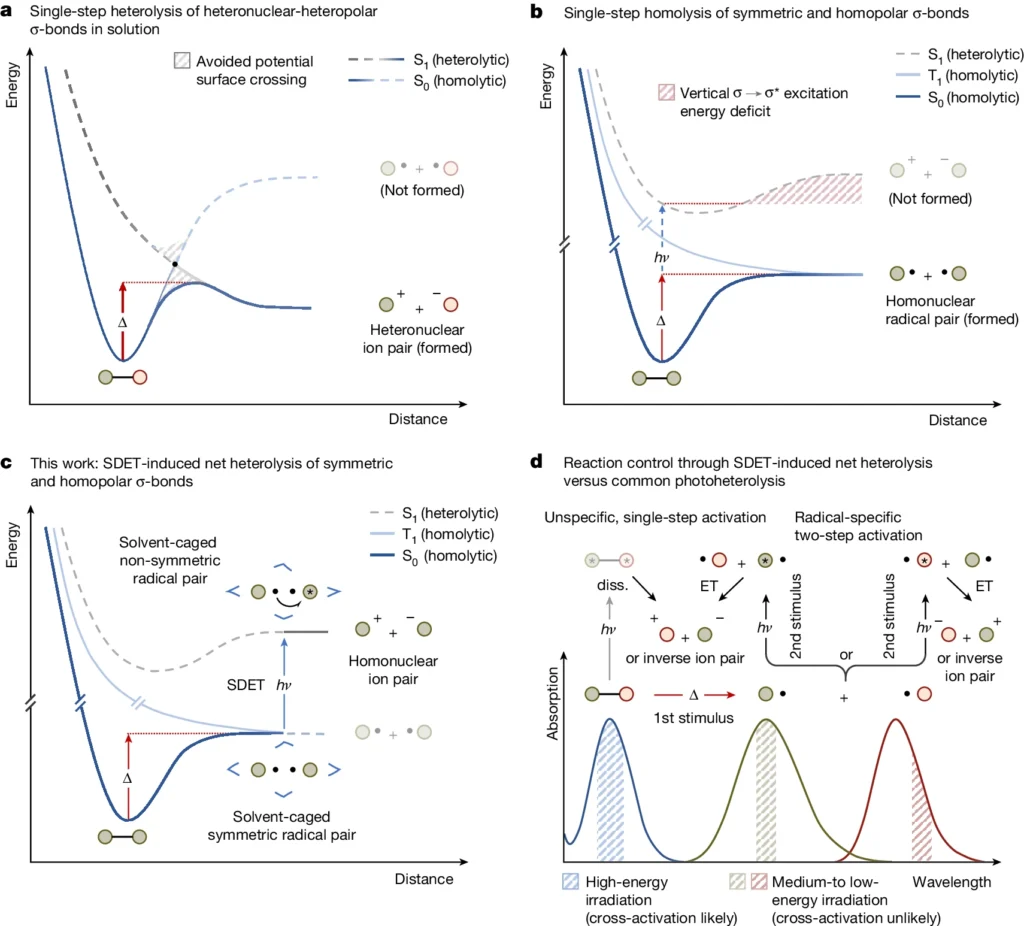
The Concept of Net Heterolysis:
The process of breaking a chemical bond and forming two different ions with opposite charges is known as net heterolysis. In reactions involving polar covalent bonds, we often observe this phenomenon, where the more electronegative atoms, the more strongly they attract the shared electrons, resulting in the formation of
Now that we have laid the groundwork for unimolecular reactions, we may proceed to explore net heterolysis, which is a particular form of bond cleavage. Heterolysis, in the field of chemistry, describes the process of breaking a chemical bond in such a way that one of the atoms acquires both electrons from the bond, leading to the creation of a cation and an anion. In contrast to homolysis, this process involves the asymmetric breaking of a bond, resulting in the formation of two radicals as each atom acquires one electron.
This process of net heterolysis is crucial for understanding how some molecules react because it creates charged species (ions) that can start other reactions or keep intermediate compounds stable. Several parameters, such as the electronegativity of the atoms and the surrounding environment (e.g., solvent polarity, temperature), influence the probability of heterolysis.
Electronegativity is a critical factor in determining which atom will get both electrons during bond cleavage. Atoms with higher electronegativity have a greater tendency to attract bonding electrons, resulting in the formation of an anion. In a compound like methyl chloride (CH3Cl), where a carbon atom binds to a chlorine atom, the more electronegative chlorine atom will take on both electrons if the bond undergoes heterolysis. This results in the formation of a chloride ion (Cl-) and a methyl cation (CH3+).
Net heterolysis is an important concept to understand in many chemical reactions, especially organic reactions that create carbocations (positively charged carbon atoms) as a common step in the middle. After they are formed, these carbocations may go through more reactions, like nucleophilic attack or rearrangement, which can make other compounds.
An Introduction to σ-Bonds:
Sigma bonds, often known as σ-bonds, are the fundamental form of covalent bonds in chemistry. Two atomic orbitals directly overlap between two atoms’ nuclei, creating a symmetrical bond along the axis connecting the two nuclei. Because of the direct overlap, -bonds are the most robust and durable form of covalent bonds, focusing the electron density between the linked atoms.
A σ-bond is formed by the sharing of a pair of electrons between two atoms, resulting in a stable and low-energy link. σ-bonds are typically the initial bonds that occur between atoms in most compounds, serving as the fundamental structure on which the remainder of the molecule is constructed. For instance, in a basic molecule such as methane (CH4), the carbon atom establishes four σ-bonds with four hydrogen atoms, resulting in the formation of a tetrahedral structure.
Nevertheless, not all σ-bonds are indistinguishable. σ-bonds can display various features, depending on the nature of the atoms involved and their relative electronegativities. The two significant types of σ-bonds that we shall concentrate on are symmetric and homopolar σ-bonds. Se–Se and C–Se σ-bond exchange experiments.
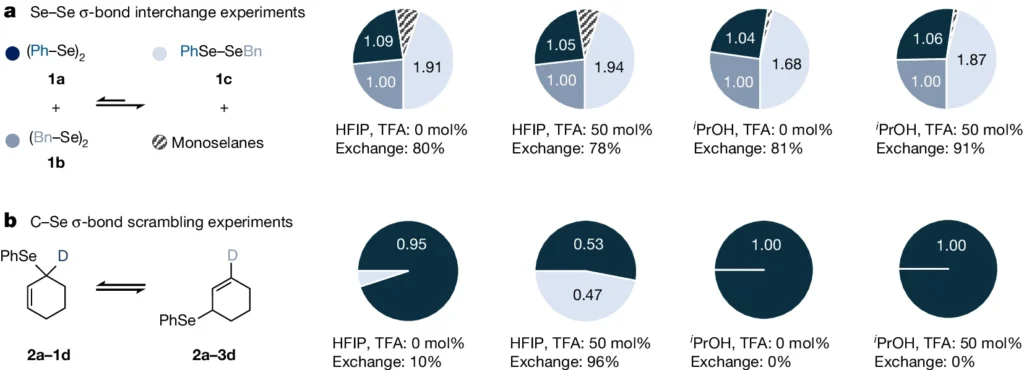
Characteristics and Behavior of Symmetric σ-Bonds:
Symmetric σ-bonds form when two atoms of the same type share electrons. Due to the atoms’ identical electronegativity, the bond’s electron density distributes evenly, preventing a permanent dipole moment. The presence of symmetry in these bonds enhances their stability and reduces their susceptibility to undergo heterolysis under typical circumstances.
Diatomic molecules such as hydrogen (H2) and nitrogen (N2) exhibit a prime illustration of a symmetrical σ-bond. In these molecules, the σ-bond is formed by overlapping identical atomic orbitals, such as 1s orbitals in H2 or sp3 orbitals in N2. This results in a binding that is both strong and difficult to break. For these symmetric σ-bonds, the bond dissociation energy, which refers to the energy needed to break the bond, is generally high, indicating their stability.
Symmetric-bonds are stable due to the uniform distribution of electrons. Because they are less likely to polarize and heterolyze. The stability of diatomic molecules, such as nitrogen, is the reason why they are not very reactive and need extreme conditions, such as high temperatures or the presence of a catalyst, to break their σ-bonds.
Homopolar σ-Bonds: Properties and Behavior
Homopolar σ-bonds, although they still contain atoms with similar electronegativity, are distinct from symmetric σ-bonds because the atoms involved are not identical. The slight disparity in electronegativity results in a minor separation of charges within the bond, causing partial charges to form on the atoms. Because homopolar π-bonds have partial charges, they are more polarized than symmetric π-bonds. This makes them more likely to break apart into different parts.
The carbon-hydrogen bond in methane (CH4) exhibits a homopolar bond. Although carbon and hydrogen have roughly identical electronegativities, there is a discernible difference that results in a tiny dipole in the bond, with carbon being slightly more electronegative than hydrogen. This weak polarization exerts an influence on the reaction of methane in varying settings.
It is most clear how reactive homopolar π-bonds are in organic chemistry, where the presence of a polar bond can affect how quickly a reaction moves forward. When there is a strong acid around, the C-H bond in methane can break apart into a methyl cation (CH3+) and a hydride ion (H-). This reaction is an essential and crucial step in numerous chemical processes, particularly those that involve carbohydrate intermediates.
Unimolecular net heterolysis refers to the breaking of symmetric σ-bonds:
The process of breaking symmetric σ-bonds through the separation of opposite charges inside a single molecule is rather uncommon because these bonds are naturally stable. Even so, symmetric π-bonds can break apart in some situations, like when there is a strong acid present, the environment is very polar, or the temperature is high.
In symmetric σ-bonds, heterolysis occurs when the bond absorbs sufficient energy to surpass the homolysis pathway. During homolysis, the bond undergoes symmetrical cleavage, resulting in the formation of two radicals as each atom acquires one electron. On the other hand, heterolysis necessitates extra energy to compel one atom to accept both electrons, resulting in the creation of a cation and an anion.
Various factors affect the probability of heterolysis occurring in symmetric σ-bonds. The bond dissociation energy is a highly influential component. The energy associated with symmetric σ-bonds tends to be high, which makes heterolysis less desirable. However, by stabilizing the resultant ions through solvation in a polar solvent, we can reduce the energy barrier for heterolysis, thereby facilitating the reaction’s progression.
The chlorine molecule (Cl2) is an example of unimolecular net heterolysis in a symmetric σ-bond. When the Cl-Cl bond is contacted by a strong acid or a polar solvent, it can break down into a chloride ion (Cl-) and a chlorine cation (Cl+). The solvent’s capacity to stabilize the resultant ions often aids in facilitating this reaction, hence increasing its thermodynamic favorability. Thermodynamic evaluation of a thermal electron transfer versus SDET between 5a• and •SePh, and transient absorption spectra of the SDET-induced ampholysis of 1a.
Unimolecular net Heterolysis refers to the process of breaking homopolar σ-bonds:
Unlike symmetric σ-bonds, homopolar σ-bonds are more prone to undergo unimolecular net heterolysis. The difference in electronegativity between the involved atoms causes the bonds to exhibit slight polarization, making them more reactive under conditions that encourage ion production.
The polarity of the bond itself makes one atom more attractive to the bonding electrons, which leads to heterolysis in homopolar π-bonds. An external factor, such as a polar solvent or the presence of a catalyst, can improve a compound’s polarity, promoting the bond’s breakage into a positively charged cation and a negatively charged anion.
The reaction between methane (CH4) and a strong acid, like hydrochloric acid (HCl), shows unimolecular net heterolysis in a homopolar π-bond. The process involves heterolysis of the C-H bond, leading to the creation of a methyl cation (CH3+) and a hydride ion (H-). The acid functions to protonate the methane, rendering the C-H bond more prone to cleavage and promoting the creation of the cation.
It’s important to know how homopolar π-bonds react in unimolecular net heterolysis because it affects organic chemistry, especially when it comes to reactions that involve carbocation intermediates. Creating carbocations through heterolysis is an important step in many chemical reactions, such as the S_N1 reaction, where making a carbocation intermediate is what slows it down.
We examine the distinctions between symmetric and homopolar bonds in the context of net heterolysis:
The primary difference between the heterolysis of symmetric and homopolar bonds lies in the differing ease of bond breaking. Symmetric σ-bonds, due to their increased stability and reduced polarization, necessitate higher energy or more extreme circumstances to perform heterolysis. On the other hand, homopolar σ-bonds, which have their polarity, are more prone to heterolysis and can undergo this process in milder conditions.
The disparity in reactivity is pivotal in the formulation of chemical reactions, especially in the realm of synthetic organic chemistry, where precise regulation of the reaction’s result is imperative. For example, when making certain organic compounds, chemists may choose to use the reactivity of homopolar π-bonds to start heterolysis and make certain intermediates or products.
It’s also important to know the difference between symmetric and homopolar π-bonds in net heterolysis to understand how stable and reactive different molecules are. Molecules that have mostly symmetrical σ-bonds tend to be more stable and less reactive. On the other hand, molecules containing homopolar σ-bonds are more likely to undergo chemical reactions, especially those that involve the creation of ions.
The process of heterolysis, where a single molecule creates two distinct products, involves thermodynamics:
The equilibrium between the bond dissociation energy and the stabilization of the resultant ions determines the thermodynamics of unimolecular net heterolysis. Heterolysis is less likely to happen with symmetric π-bonds because the bond dissociation energy is high. This is because the ions that are formed need to be strongly stabilized, which can be done by solvating them in a polar solvent.
The bond dissociation energy (BDE) refers to the amount of energy needed to break a σ-bond and create ions. The energy of symmetric σ-bonds is generally large because of the robust and stable character of the bond. However, the surrounding environment can effectively support the ions produced during heterolysis, thereby reducing the total energy required for the reaction.
Because homopolar π-bonds have lower bond dissociation energy than symmetric π-bonds, heterolysis is more likely to happen. The weak polarization of homopolar σ-bonds results in a partial charge separation. Solvation or other mechanisms can further maintain this, thereby lowering the energy barrier for bond breaking.
Entropy and enthalpy play significant roles in the thermodynamics of unimolecular net heterolysis. Entropy, which quantifies the level of chaos in a system, often rises when a molecule undergoes heterolysis, as the creation of two distinct ions results in a more disorganized state. On the other hand, enthalpy signifies the system’s heat content and closely correlates with the energy needed to break chemical bonds. When strong β-bonds break, enthalpy rises dramatically. This renders the process less desirable unless it is counterbalanced by beneficial increases in entropy or other stabilizing factors.
Temperature and solvent conditions heavily influence the thermodynamics of unimolecular net heterolysis. High temperatures can supply the necessary energy to overcome the bond dissociation energy, while polar solvents can stabilize the formed ions, thereby increasing the likelihood of the reaction. By exerting control over these parameters, chemists can regulate the circumstances in which heterolysis takes place, optimizing reactions to achieve desired results.
The investigation focuses on the speed at which a solitary molecule experiences heterolysis, leading to the creation of two distinct products:
The rate at which a bond cleaves and the factors influencing this rate are known as the kinetics of unimolecular net heterolysis. Symmetric σ-bonds exhibit a high bond dissociation energy, which leads to slower reaction rates due to the increased energy needed to reach the transition state. In contrast, homopolar σ-bonds, due to their lower bond dissociation energy, demonstrate higher rates of heterolysis.
The activation energy required to break the bond determines the pace of unimolecular net heterolysis. The activation energy is the lowest amount of energy required to start a reaction and achieve the transition state. Generally, symmetric bonds have a large activation energy, signifying their stability and the significant energy barrier they must overcome.
Conversely, the activation energy required for homopolar σ-bonds is typically lower, resulting in accelerated reaction rates. Other substances, such as a catalyst or a polar solvent, can more easily break these naturally polar chemical bonds, thereby reducing the activation energy and accelerating heterolysis.
Spectroscopy and mass spectrometry are experimental techniques used to explore the kinetics of unimolecular net heterolysis. These techniques allow scientists to see the formation of ions in real time and quantify the speed at which bond breaking occurs. Spectroscopy provides valuable insights into a reaction’s kinetics by examining changes in light absorption or emission during its progression. Mass spectrometry, in contrast, enables the determination and measurement of the ions produced during heterolysis, offering precise insights into the rate and mechanism of the reaction.
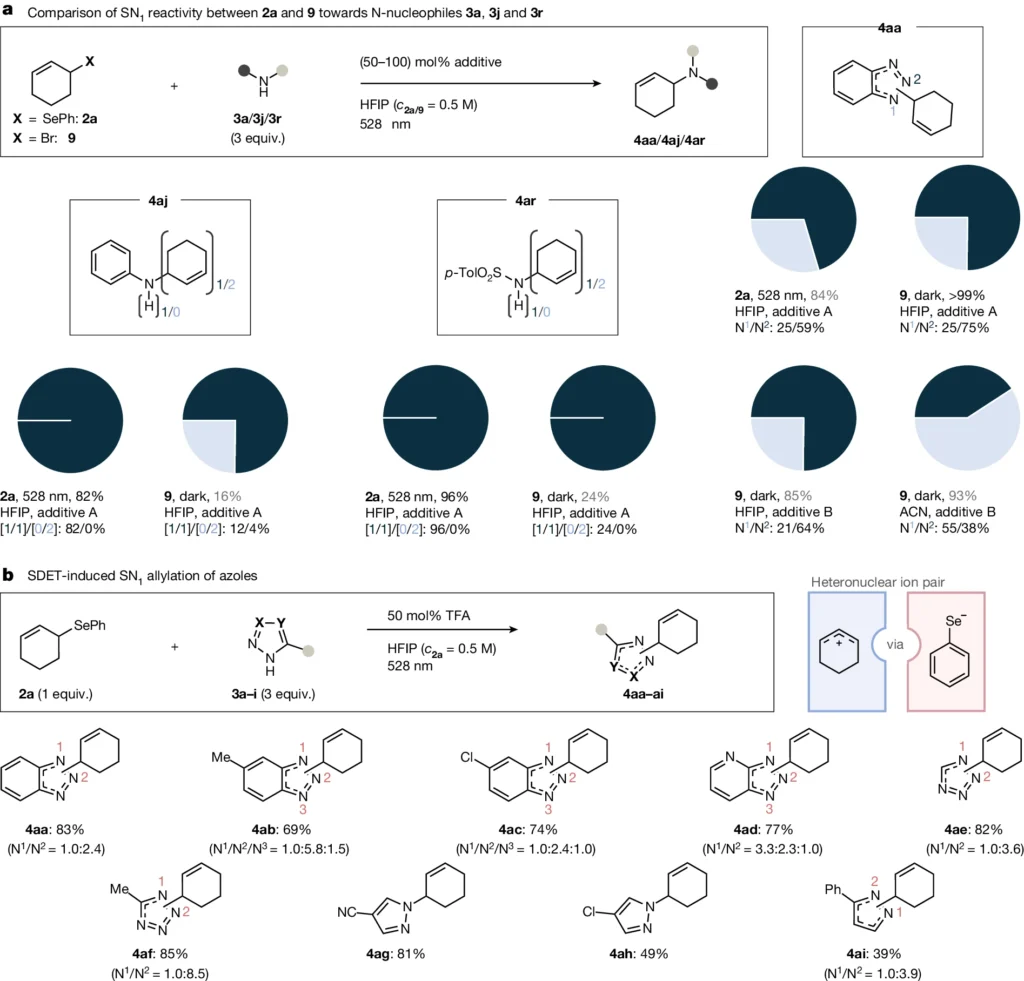
The Arrhenius equation illustrates the relationship between the reaction rate, activation energy, and temperature. We can use it to describe the kinetics of unimolecular net heterolysis. Chemists can gain a more profound comprehension of the components that affect a reaction by examining the rate data acquired from experiments, chemists enable them to determine the activation energy and other kinetic parameters. Comparative reactivity study of 2a versus 9 and SDET-induced atypical SN1 amination of allyl- and alkyl(aryl)selanes.
There are strategies for investigating net heterolysis in experiments:
Precise experimental methods are required to accurately see and measure the unimolecular net heterolysis of the -bonds process. Typical techniques comprise spectroscopy, mass spectrometry, and computational chemistry.
Spectroscopy employs various techniques, such as UV-Vis, IR, and NMR spectroscopy, to observe alterations in molecule structure and the generation of ions during heterolysis. These approaches provide instantaneous information on the reaction’s progress and the intermediates it produces.
Mass spectrometry enables the detection and measurement of ions generated during heterolysis, facilitating their identification and quantification. Chemists can determine the composition of the reaction products and acquire knowledge about the reaction mechanism by quantifying the mass-to-charge ratio of the ions.
In computational chemistry, methods like quantum chemical calculations and molecular dynamics simulations are used to model and study the reaction paths and potential energy surfaces of unimolecular net heterolysis. These simulations offer comprehensive insights into the electrical configuration of the molecules and the variables that impact the reaction.
Although these techniques are effective for investigating net heterolysis, they also present difficulties. For instance, it might be challenging to separate and analyze the impacts of particular factors, such as temperature or solvent effects, in intricate systems. Moreover, understanding the reaction mechanism in experimental data typically necessitates the integration of both experimental and computational methodologies.
Understanding the process of unimolecular net heterolysis has far-reaching implications in a variety of chemistry fields. In organic synthesis, it is important to be able to control the cleavage of β-bonds caused by heterolysis to products and intermediates that are wanted. Chemists can achieve outstanding selectivity and efficiency in reactions by adjusting the circumstances that lead to heterolysis.
Uses of Unimolecular Net Heterolysis:
Industrial processes like polymerization and catalysis apply the principles of unimolecular net heterolysis to improve reaction conditions and boost yields. When certain monomers are polymerized, for example, being able to control the heterolysis of π-bonds could lead to the formation of certain polymer architectures that have good properties.
Controlling the process of unimolecular net heterolysis has significant ramifications for the fields of chemical engineering and materials research. Engineers can optimize production procedures by comprehending the aspects that impact bond cleavage, resulting in enhanced efficiency and reduced waste. This, in turn, promotes sustainability and cost-effectiveness.
Theoretical models and computational studies:
Theoretical models and computational research are essential for comprehending the mechanisms and factors that impact unimolecular net heterolysis. Quantum chemical methods, like density functional theory (DFT), help us fully understand how molecules’ electrons are arranged and the potential energy landscapes that come with heterolysis.
Chemists can use computational simulations to accurately describe the reaction pathways of unimolecular net heterolysis. This enables them to anticipate reaction outcomes and identify the critical components that influence the process. Additionally, they can use these simulations to explore the effects of different solvents, temperatures, and catalysts on the reaction, providing valuable insights for optimizing experimental settings.
Computational simulations of specific cases have offered valuable insights into the impact of substituents on the molecule and the influence of solvent effects on heterolysis. Simulations have shown that adding electron-withdrawing groups can increase the chance of heterolysis by making the ions that form more stable. Similarly, the choice of solvent can greatly influence the course of a reaction, as polar solvents tend to promote heterolysis, whereas non-polar solvents tend to favor homolysis.
When you combine theoretical models, computational studies, and experimental data, you can fully understand unimolecular net heterolysis. This gives you useful information for both basic research and real-world applications.
In conclusion:
It is hard and interesting to study how symmetric and homopolar π-bonds break apart in a single molecular system. Gaining knowledge about the mechanisms, thermodynamics, and kinetics of these reactions offers a significant understanding of the stability and reactivity of various molecules, which has wide-ranging consequences for organic synthesis, industrial processes, and chemical engineering.
The different ways that symmetric and homopolar π-bonds can be broken down by heterolysis show how important molecular structure and electronegativity are in figuring out how reactions happen. Chemists can achieve great selectivity and efficiency in reactions. This ability allows for the creation of new materials, medicines, and industrial processes.
Continued research on this topic will undoubtedly result in new findings and advancements in chemical science by enabling the manipulation of unimolecular net heterolysis. This will offer novel tools and approaches for comprehending and managing chemical reactions.
Frequently Asked Questions:
1). The distinction between unimolecular and bimolecular reactions lies in the number of molecules involved in each type of reaction.
Unimolecular reactions are chemical changes that occur in a single molecule, usually due to its internal energy. Bimolecular reactions include the interaction of two molecules, resulting in the creation of products.
2). What is the effect of electronegativity on σ-bond heterolysis?
Electronegativity determines the allocation of electrons within a chemical bond. Heterolysis is a process where the more electronegative atom is more likely to attract the bonding electrons, leading to the creation of an anion. The disparity in electronegativity between linked atoms can also generate a dipole, rendering the bond more susceptible to heterolysis.
3). What are some common examples of symmetric and homopolar σ-bonds?
Symmetric bonds form between identical atoms in diatomic molecules like hydrogen (H2) and nitrogen (N2). Homopolar σ-bonds form between atoms with comparable electronegativity but are not identical, like the C-H bond in methane (CH4).
4). How do solvents affect the rate of unimolecular net heterolysis?
Solvents can significantly influence the speed of unimolecular net heterolysis by stabilizing the ions generated during the reaction. Polar solvents captivate energy and enhance the reaction rate by stabilizing the resultant cation and anion.
5). Is unimolecular net heterolysis appropriate for drug synthesis?
Indeed, unimolecular net heterolysis plays a critical role in organic synthesis, including drug manufacturing. By controlling heterolysis, chemists can selectively break certain bonds, resulting in the creation of desired intermediates and products. The process of selectively breaking chemical bonds is essential for the production of intricate organic compounds, such as medications.
For more chemistry blogs, visit chemistry Master