Table of Contents
Preface:
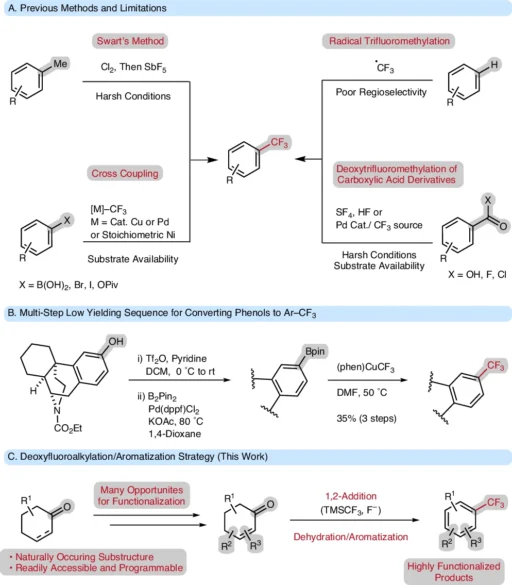
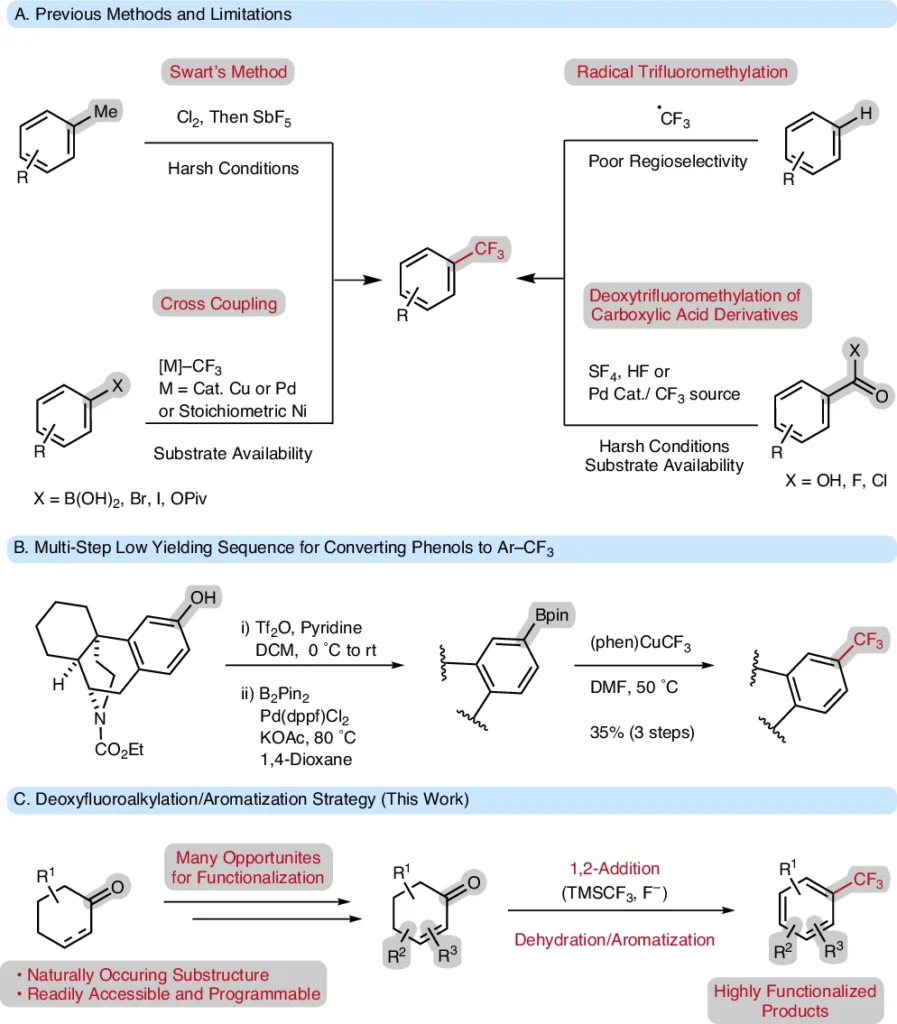
There has been a growing interest in trifluoromethyl groups (-CF) throughout the chemical community, attributable to their distinctive chemical and physical characteristics. These groups are widely used in research on medicines, agrochemicals, and materials. Changing cyclohexanone and cyclohexenone derivatives into highly substituted trifluoromethyl arenes is an important study area because they are excellent starting materials for synthetic chemistry. After aromatization, the deoxy trifluoromethylation process gives a direct way to trifluoromethyl arenes, combining efficiency with variety.
This article will examine the deoxytrifluoromethylation/aromatization process, detailing its mechanics, advantages, uses, and the obstacles encountered in practical implementation. Common methods to access trifluoromethylarenes.
Comprehension Trifluoromethylation:
Trifluoromethylation denotes the incorporation of a trifluoromethyl group (CF) into a molecule. This functional group is essential for enhancing the chemical stability, lipophilicity, and bioavailability of organic molecules. As a result, it has gained extensive use, particularly in drug discovery, where its integration into pharmaceutical compounds may improve metabolic stability and increase binding affinity to target proteins.
Numerous blockbuster pharmaceuticals, including fluoxetine (Prozac) and celecoxib (Celebrex), have trifluoromethyl groups. The CF₃ group’s extensive applicability in pharmaceutical and materials chemistry makes its incorporation into organic molecules a critical aspect of contemporary organic synthesis.
Cyclohexanones: A Concise Overview
Because they can serve as flexible building blocks for many functionalized aromatic compounds, cyclohexanone, and cyclohexenones are important steps in the synthesis of many chemicals. Cyclohexanone is a six-membered ring ketone that can go through many reactions, such as reduction, oxidation, and substitution. This makes it useful for making complex compounds. Cyclohexenone, a conjugated ketone, is also very flexible and works well in processes like nucleophilic addition and cyclization.
Both structures can undergo aromatization, rendering them suitable for processes that incorporate trifluoromethyl groups.
The Concept of Deoxytrifluoromethylation:
Deoxytrifluoromethylation is a procedure that entails removing an oxygen atom from a substrate while simultaneously introducing a trifluoromethyl group. This technique is particularly advantageous for cyclohexan(en)ones since it facilitates the direct conversion of saturated or partially unsaturated rings into aromatic systems.
Adding the CF₀ group not only makes the molecule better, but it also speeds up the aromatization process. This turns the six-membered ring into an aromatic compound and creates highly substituted trifluoromethyl arenes.
Aromatization in Organic Chemistry:
Aromatization is the process of transforming a non-aromatic chemical into an aromatic compound. The cyclic structures of aromatic compounds, which include delocalized π-electrons, give them stability and unique chemical properties. In deoxy trifluoromethylation, aromatization generally occurs after the incorporation of a trifluoromethyl group, facilitating the synthesis of stable, highly substituted trifluoromethyl arenes. The deoxytrifluoromethylation/aromatization strategy tolerates many useful functional groups and substitution patterns.
Mechanistic Insights on Deoxytrifluoromethylation and Aromatization:
The activation of the cyclohexane (en)one substrate initiates the deoxy trifluoromethylation process. Usually, a catalyst or the appropriate reagent facilitates the joining of the trifluoromethyl group. As soon as the CF group is added, the molecule goes through a series of electron rearrangements that get rid of an oxygen atom, usually from a carbonyl group, and create an entirely aromatic ring structure.
Transition metal complexes serve as catalysts, significantly reducing activation energy and enhancing reaction rates in these reactions. Specific reaction parameters, such as solvent choice, temperature, and pressure, can have a greater impact on the reaction’s efficiency and selectivity.
Conditions for Deoxytrifluoromethylation Reactions:
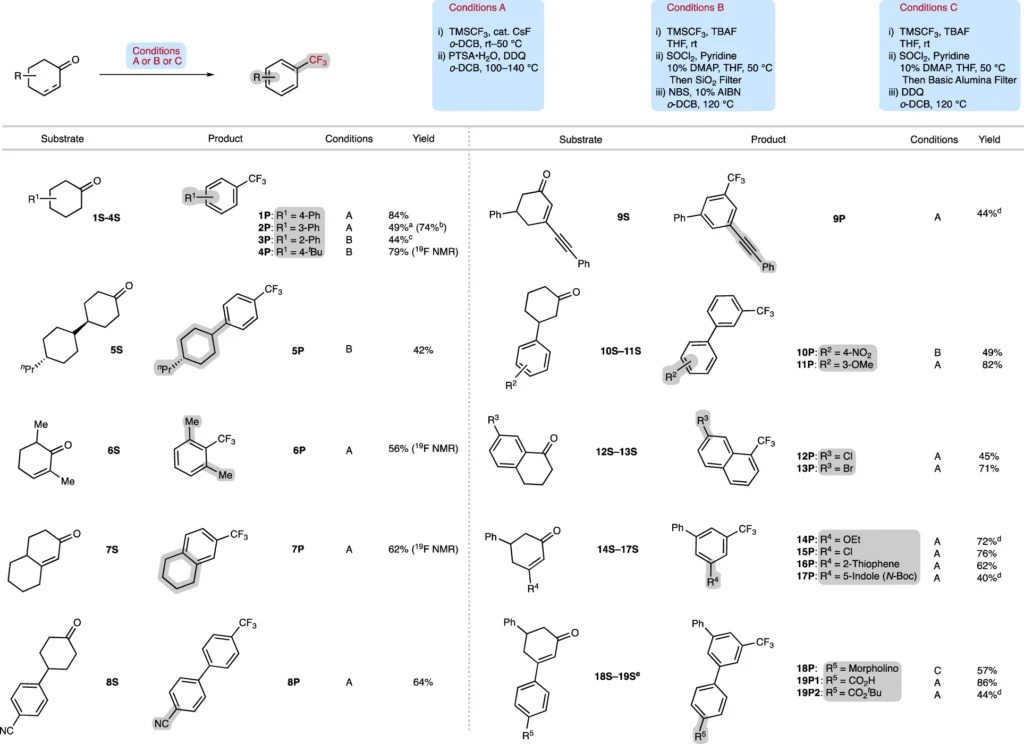
For instance, the deoxytrifluoromethylation/aromatization process requires trifluoromethylating agents such as Togni’s reagent or Ruppert-Prakash reagent (TMS-CF). Reaction temperatures may fluctuate; however, relatively increased temperatures (about 80°C to 150°C) are frequently required to facilitate the aromatization process. We frequently employ solvents like dichloromethane or acetonitrile to dissolve the reactants and promote the reaction.
Catalysts Participating in the Reaction:
The choice of catalyst is critical to the efficacy of deoxytrifluoromethylation processes. Palladium, nickel, and copper complexes serve as catalysts, each offering distinct advantages for reaction scope and selectivity. Ligands used in these catalytic systems can influence the reaction outcome by stabilizing the metal center or directing the reaction pathway, enhancing yields and selectivity.
Substrate range and functional group compatibility:
The deoxytrifluoromethylation/aromatization process is characterized by its extensive substrate scope. This change can occur in cyclohexanones and cyclohexenones with different functional groups, such as alkyl, aryl, and heteroatom substituents. Moreover, the technique accommodates a variety of functional groups, including esters, nitriles, and halogens, rendering it a flexible instrument in organic synthesis.
Utilizations of Trifluoromethyl Arenes:
Trifluoromethyl arenes are required in a variety of industries. Because of their bioactive characteristics, they serve as fundamental structures for numerous medications in pharmaceuticals. Agrochemicals improve the effectiveness and environmental durability of pesticides and herbicides. In materials science, trifluoromethyl arenes facilitate the advancement of sophisticated polymers and other useful materials.
Benefits of the Deoxytrifluoromethylation/Aromatization Process:
This approach has many advantages, including great selectivity, efficiency, and the capability to produce highly substituted trifluoromethyl arenes with few side reactions. This procedure is frequently more environmentally sustainable than alternative trifluoromethylation processes, producing less detrimental by-products. The deoxytrifluoromethylation/aromatization strategy pushes the limits of accessible Ar–CF3 compounds.
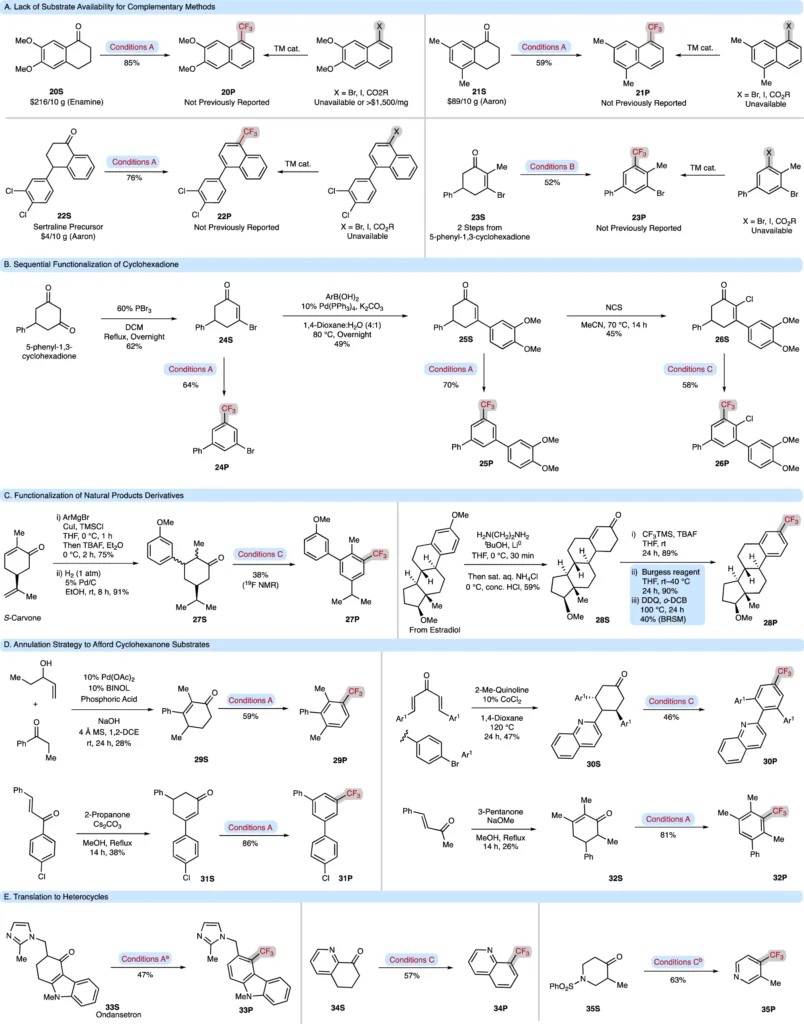
Obstacles and Constraints:
Notwithstanding its numerous benefits, the deoxytrifluoromethylation/aromatization method encounters certain obstacles. Regulating reaction conditions, particularly temperature and pressure, can be challenging. Furthermore, certain substrates may exhibit incompatibility with the employed catalysts, hence constraining the reaction’s scope in specific instances.
Recent developments in the discipline:
Recent studies have focused on expanding the types of substrates that can be used, improving the efficiency of the catalytic process, and making new trifluoromethylating agents that are more environmentally friendly and cost-effective. Advancements in this domain are addressing the constraints of previous techniques, enhancing the process’s accessibility for diverse applications.
Prospective Outlooks:
Future investigations may look into the development of more adaptable catalytic systems as well as the use of environmentally friendly solvents and reagents. Moreover, broadening the reaction’s breadth to encompass a wider array of substrates, including heterocyclic molecules, will augment its applicability in synthetic chemistry.
Summary:
Deoxytrifluoromethylation, succeeded by aromatization, is a highly effective pathway to get trifluoromethyl arenes. These compounds hold considerable significance in research on medicines, agrochemicals, and materials. The process is discerning, and effective, and offers potential for additional innovation. As research in this domain advances, we anticipate larger applications and enhancements in the field.
Frequently Asked Questions:
1. What is deoxytrifluoromethylation?
A chemical reaction called deoxytrifluoromethylation takes out an oxygen atom from a molecule and adds a trifluoromethyl (CF₃) group in its place.
2. What are the primary obstacles to this reaction?
Challenges encompass regulating reaction conditions and verifying the substrate’s compatibility with the selected catalyst.
3. What is the significance of trifluoromethyl arenes?
Trifluoromethyl arenes are extensively utilized in medicines, agrochemicals, and materials research owing to their improved stability and bioactivity.
4. Is this approach scalable for industrial applications?
Indeed, we can scale this process with appropriate optimization, but it requires meticulous regulation of reaction parameters.
5. What future investigations are required in this domain?
Future research will likely concentrate on broadening the variety of substrates, enhancing catalyst efficiency, and advancing process sustainability.
For more chemistry blogs, visit chemistry Master