Table of Contents
Acidic Water Oxidation is an essential step in generating clean energy, particularly for technologies such as solar fuels and electrolyzers. This reaction’s efficacy under acidic conditions poses significant challenges. A viable strategy entails using dual-active site catalysts to improve the oxide route mechanism’s efficacy. This article looks at how operando techniques help scientists figure out these processes in real time and explains how the oxide pathway helps acidic water oxidation.
Comprehending the Acidic Water Oxidation:
Water oxidation, also known as the oxygen evolution reaction (OER), entails the dissociation of water molecules to generate oxygen gas, electrons, and protons. This half-reaction is crucial in operations such as water splitting for hydrogen generation. Although OER can take place in both acidic and basic settings, the acidic environment presents distinct problems owing to the medium’s corrosive properties. So, finding stable and effective catalysts for oxidizing acidic water is important for the progress of energy storage technologies, such as proton-exchange membrane (PEM) electrolyzers.
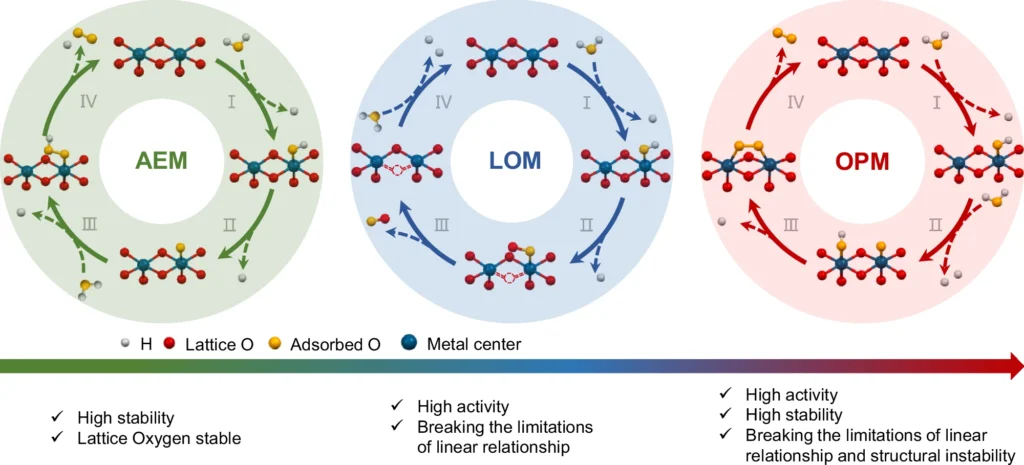
An Overview of the Oxide Path Mechanism:
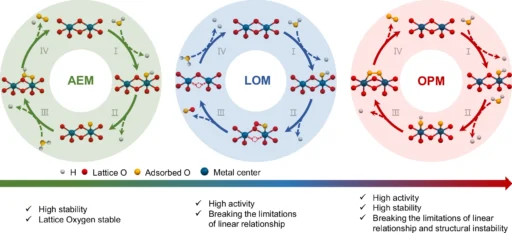
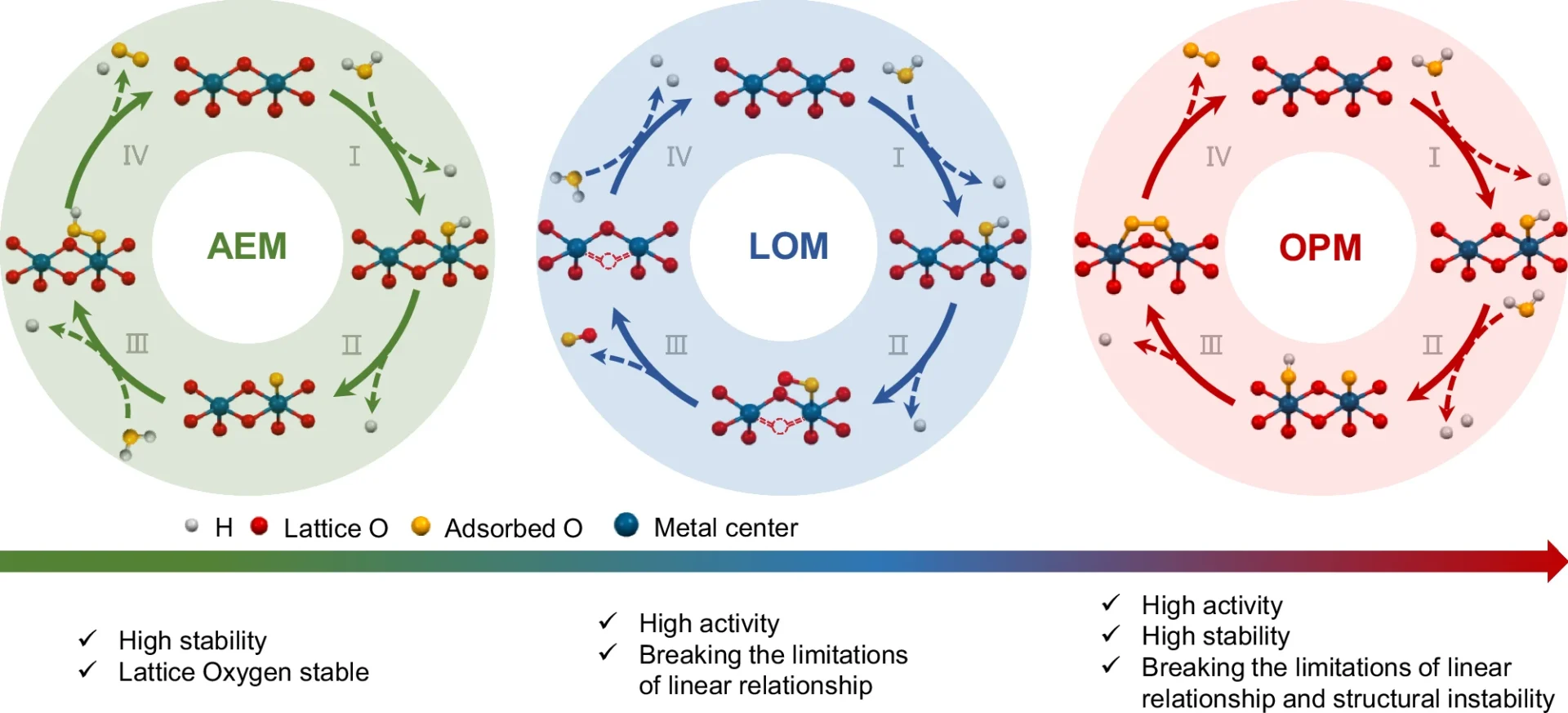
In electrocatalytic water oxidation, the oxide path mechanism is critical. This method involves the generation of oxide species on the catalyst surface, which participate in a sequence of redox events leading to the production of oxygen gas. The process encompasses several stages, including water adsorption, hydroxide species production, and the eventual liberation of oxygen. The oxide pathway’s efficacy is primarily dependent on the catalyst’s capacity to generate and stabilize these oxide species.
What are dual-active sites?
Dual-active sites denote catalytic systems in which two different types of active centers collaborate to augment the catalytic process. In these places, there is usually a metal atom connected to an oxide species. This makes it easier for electrons to move around and keeps the intermediates stable during the process. When it comes to acidic Water oxidation, dual-active sites improve reaction kinetics more than single-active sites do by making it easier to make and break down important intermediates.
Mechanistic insights into the oxygen pathway:
The oxide path process begins with water molecules adsorbing onto the catalyst surface. These molecules undergo a sequence of electron transfer processes, resulting in the generation of oxygen intermediates. Ultimately, the catalyst surface expels O2, the result of these intermediates amalgamating. In dual-active site catalysts, the metal center usually speeds up the adsorption and first oxidation processes, while the oxide species keeps the oxygen intermediates stable, which makes the whole reaction more effective. OER mechanism analysis.
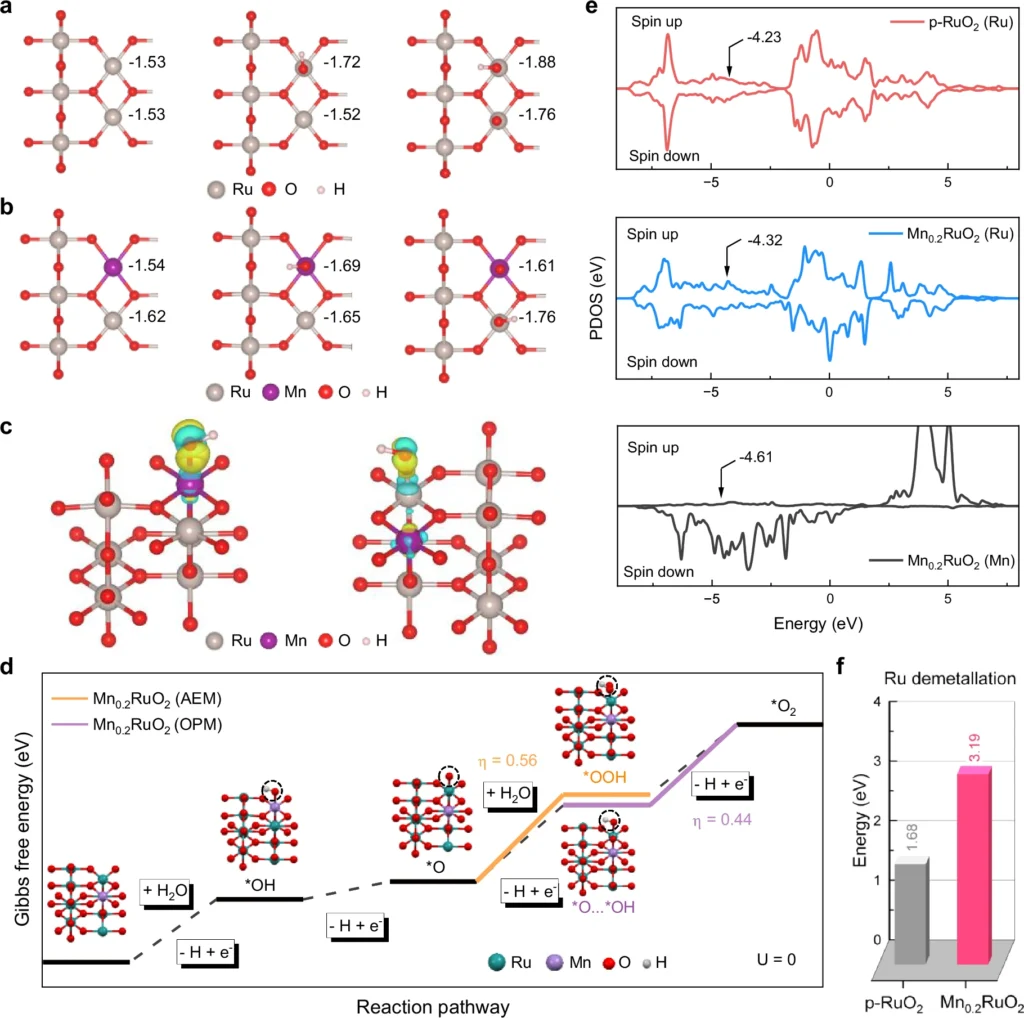
Function of Oxides in Water Oxidation Catalysis:
We recognize oxides, especially transition metal oxides like ruthenium oxide (RuO2) and iridium oxide (IrO2), for their stability and efficacy in water oxidation. These oxides can provide a stable foundation for the production of oxygen intermediates, which is critical for the oxide route mechanism’s efficacy. Under acidic environments, where other substances may deteriorate, oxides preserve their structural integrity, facilitating ongoing oxygen evolution.
Impact of Dual-Active Sites on the Oxide Pathway:
The existence of dual-active sites significantly enhances the oxide pathway’s efficacy. When a metal center and an oxide combine, the metal initiates the first water-splitting phase, while the oxide stabilizes the oxygen intermediates. The synergistic interaction between the two active sites results in accelerated reaction kinetics and enhanced total catalytic activity. In operando investigations, researchers have noted that dual-active sites diminish the overpotential necessary for acidic Water oxidation, enhancing the energy efficiency of the process.
Experimental Techniques for Operando Identification:
Operando approaches allow researchers to observe catalytic reactions in real time in authentic working environments. This is essential for comprehending dynamic processes such as the oxide route mechanism in water oxidation. X-ray absorption spectroscopy (XAS) and in-situ electrochemical analysis help us understand how the structure and electricity of the catalyst surface change during the reaction. Employing operando methods, researchers can directly study the function of dual-active sites and their influence on the reaction pathway. Fine-structure characterization of Mn0.2RuO2.
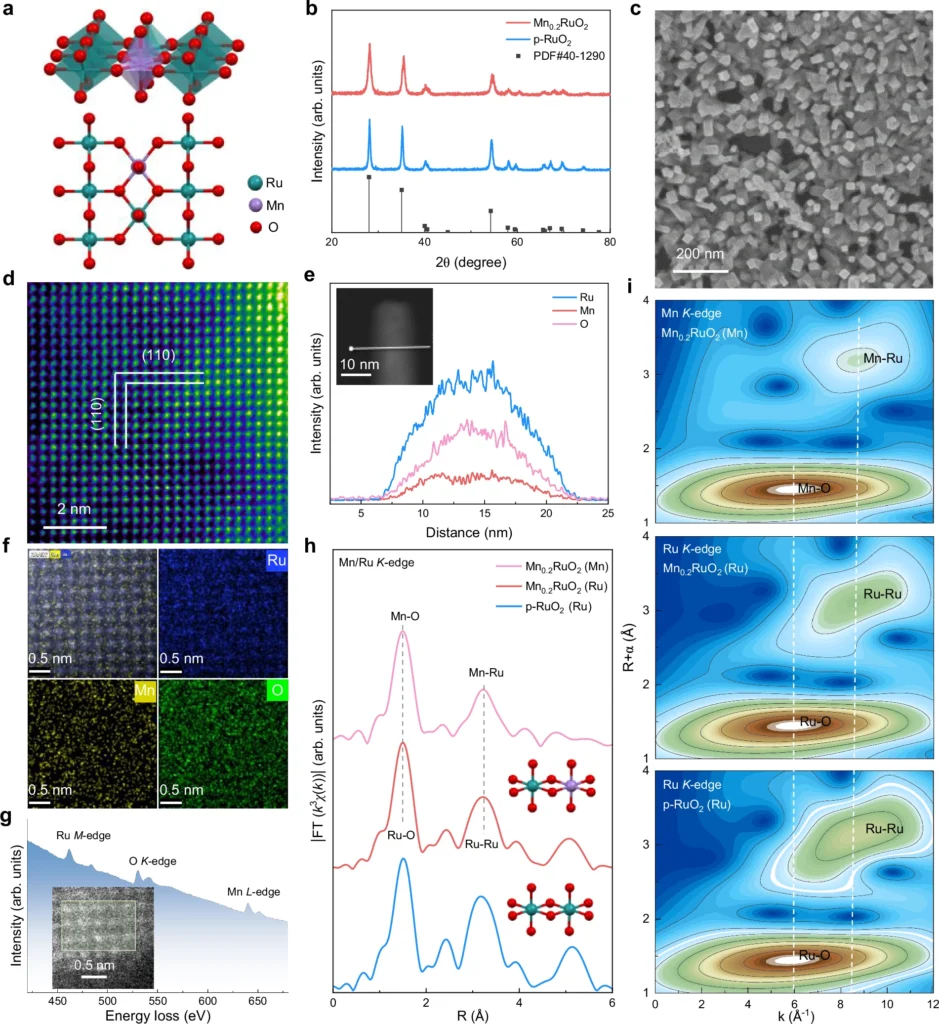
Case Studies: Dual-Active Site Catalysts
Numerous studies have proven the efficacy of dual-active site catalysts in acidic water oxidation. Catalysts composed of iridium-ruthenium oxides have exceptional stability and activity. Operando XAS studies have shown that these two active sites make it easier for active oxygen species to form, which leads to better OER performance. Cobalt-iron oxide complexes exhibit enhanced catalytic activity due to the synergistic interaction between cobalt and iron centers.
Obstacles in Examining the Oxide Path:
Despite the advancements in operando methodologies, challenges continue to arise when examining the oxide path mechanism. A significant concern is the stability of catalysts in acidic environments, as numerous materials deteriorate over time. The complexity of dual-active site interactions makes it difficult to isolate each site’s specific part in the response process. Inconsistencies in results may arise due to variability in experimental circumstances and catalyst preparation.
Benefits of Operando Techniques in Mechanistic Identification:
Operando approaches offer numerous advantages over traditional post-reaction analysis. These technologies facilitate the acquisition of real-time data, enabling researchers to document the dynamic alterations that transpire during the reaction. This is especially crucial for intricate systems like the oxide pathway, where intermediates can develop and disintegrate swiftly. Operando approaches have transformed the catalysis domain by providing enhanced insights into reaction pathways and catalyst performance. Activity and stability measurement.
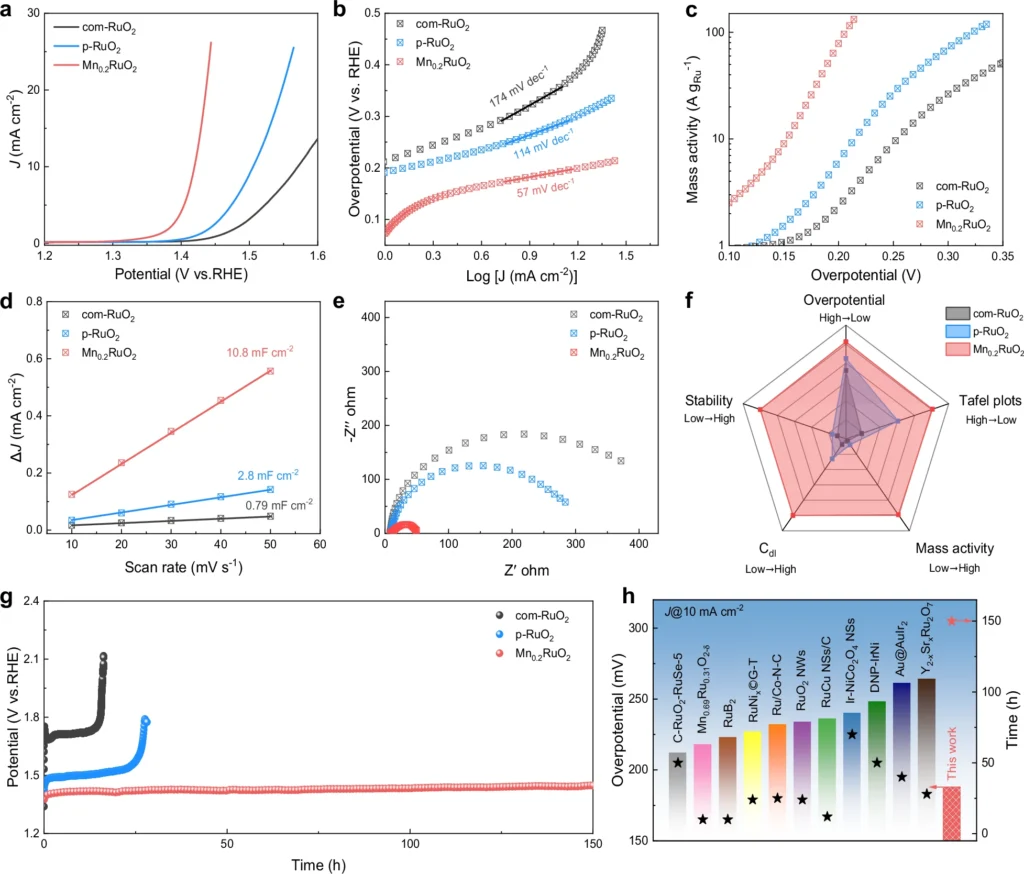
Enhancing dual-active site catalysts:
Researchers are investigating diverse techniques to improve the efficacy of dual-active site catalysts. One method involves designing catalysts with enhanced metal-oxide interactions to augment the stability and efficacy of the active sites. Another technique involves creating materials with increased surface areas, facilitating a greater number of active sites to engage in the reaction. These enhancements may result in more efficient acidic Water oxidation systems, especially in acidic conditions.
The significance of theoretical calculations:
Theoretical calculations, like those employing Density Functional Theory (DFT), are essential in correlating experimental results. DFT studies can predict the performance of various catalyst designs in water oxidation by predicting the interactions between metal and oxide centers. These calculations improve operando studies by giving a molecular-level understanding of how the reaction works, which helps researchers make better catalysts.
Prospective Avenues for Research on Acidic Water Oxidation:
Future advancements in materials for acidic water oxidation will likely emphasize the creation of more resilient dual-active site systems. The advancement of operando techniques will continue to elucidate the oxide route mechanism, facilitating the development of more efficient catalysts. Furthermore, the amalgamation of computational and experimental methodologies will expedite the identification of novel materials for energy conversion applications.
Final Assessment:
The operando identification of the oxide route mechanism has shed light on the importance of two active sites in acidic Water oxidation. Researchers are elucidating the fundamental parameters that dictate catalytic performance by integrating experimental methodologies with theoretical insights. As we progress towards a future of sustainable energy, ongoing research in this domain will be essential for creating more efficient and resilient catalysts.
Frequently Asked Questions:
1). What is the importance of dual-active sites in acidic Water oxidation?
Dual-active sites make catalysis more effective by letting two different active centers work together. This speeds up the reaction and keeps important intermediates stable.
2). What distinguishes operando techniques from conventional methods?
Operando approaches provide real-time observation of reactions under genuine operating settings, offering insights into dynamic alterations that post-reaction analysis fails to capture.
3). What are the primary challenges associated with using oxides in acidic Water oxidation?
Oxides may deteriorate in severe acidic environments, and identifying materials that sustain stability while delivering high activity presents a significant problem.
4). What is the significance of the oxide path mechanism?
The oxide route mechanism is important for oxygen evolution because it creates and stabilizes the oxygen intermediates that are needed to split water.
5). In what ways does computational research enhance operando findings?
Computational investigations, such as DFT, offer a molecular-level comprehension of catalyst action, facilitating the explanation and prediction of experimental outcomes derived from operando techniques.
For more chemistry blogs, visit chemistry Master