Table of Contents
A brief overview of Poly(3,4-ethylenedioxythiophene) :
In the world of advanced materials, conducting polymers stand out as unique compounds that combine the properties of plastics with electrical conductivity, a feature typically associated with metals. Poly(3,4-ethylenedioxythiophene), often known as PEDOT, is a highly researched and extensively used electronic conducting polymer. Its outstanding electrical conductivity, along with its amazing transparency in thin films, chemical stability, and ease of production, make it a valuable material in modern technology. Organic electronics, solar cells, supercapacitors, and biosensors in particular use PEDOT.
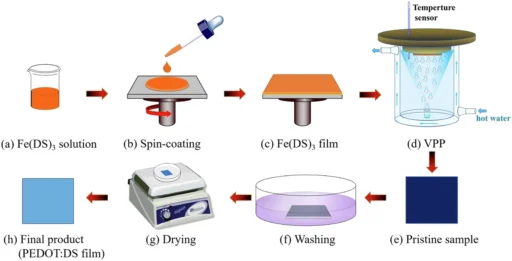
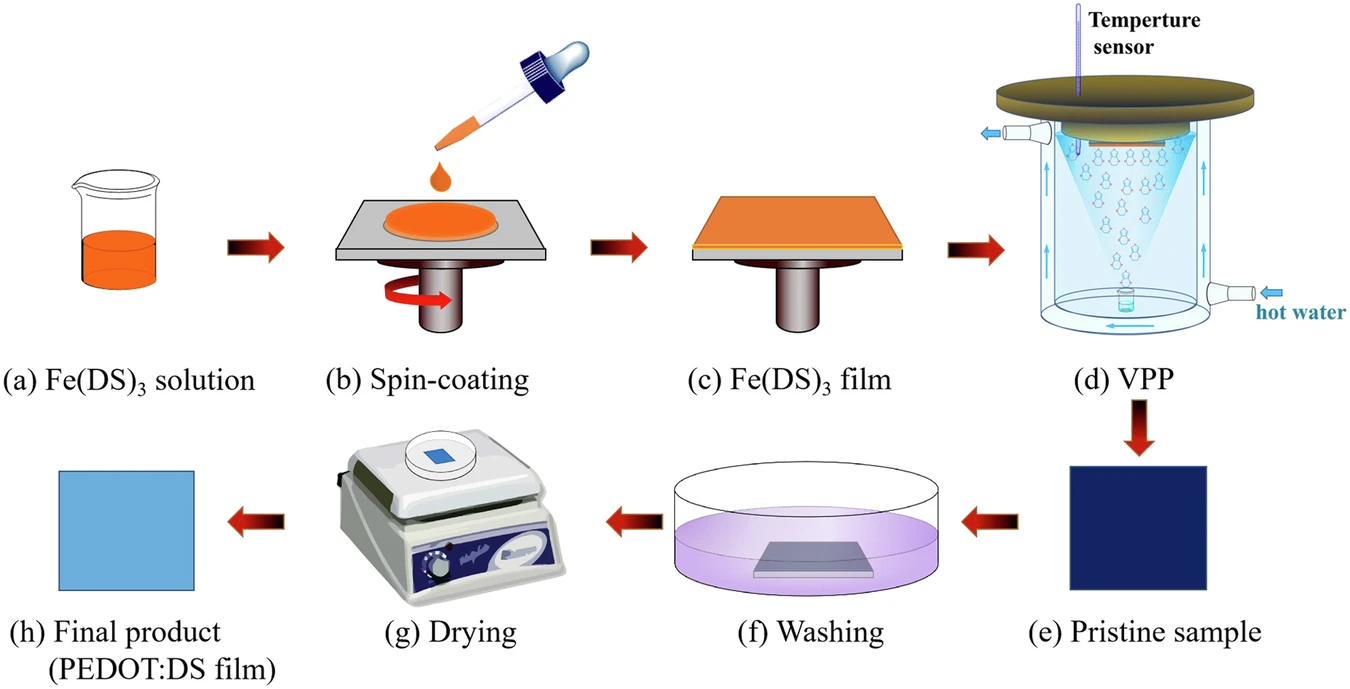
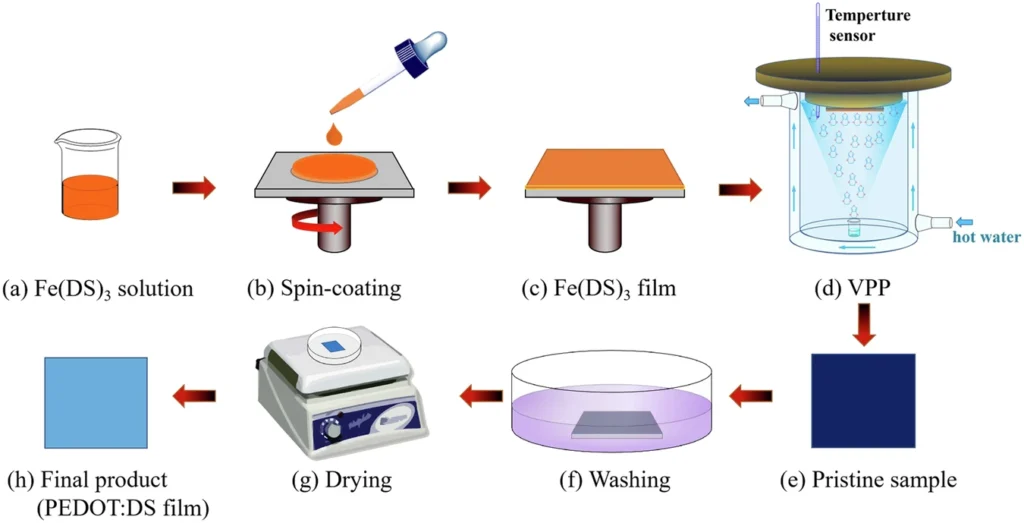
Like any other material, PEDOT also has the potential to improve its performance. To achieve this, one must regulate its crystallinity, which directly affects its conductivity and mechanical characteristics. The presence of iron(III) dodecyl sulfate enables the complex phenomenon of directed crystallization in PEDOT films, leading to the subsequent development of a lamellar metastructure. The reported method significantly enhances PEDOT’s performance, thereby offering new prospects for its application. Preparation of VPP-PEDOT.
Crystallization of Conductive Polymers: A Critical Analysis
The process of crystallization involves organizing atoms, ions, or molecules in a repeating pattern to create a highly structured structure. Achieving a crystalline structure is an essential aspect of optimizing the performance of conducting polymers, particularly in terms of charge transfer.
The electrical conductivity of most polymers, including PEDOT, can be significantly dependent on the chain alignment and crystallinity. Polymer crystalline domains facilitate enhanced charge mobility through improved alignment of polymer chains, reducing the obstacles for electrons or holes to traverse. In devices such as organic transistors, ensuring charge mobility is critical because it directly affects both speed and efficiency. In contrast, a greater degree of amorphous structure (characterized by disordered areas) frequently results in decreased conductivity and diminished mechanical stability.
Although PEDOT has a well-established conductivity, manipulating its crystallinity can significantly enhance its performance. The challenge lies in the fact that achieving controlled and homogeneous PEDOT crystallization is far from straightforward. That is when the process of directed crystallization and the involvement of specific additives, such as iron(III) dodecyl sulfate, become relevant.
Intricacies of PEDOT Crystallization:
PEDOT naturally forms amorphous films, characterized by randomly arranged molecules lacking long-range organization. This is a significant challenge, especially for thin films, which find widespread use in electronics and energy storage applications. Thin films are intrinsically more prone to flaws and irregularities during the crystallization process, which can greatly diminish their effectiveness.
Numerous factors contribute to the challenge of crystallizing PEDOT.
Chain rigidity: The polymer chains of PEDOT exhibit a restricted level of flexibility, which hinders their ability to conform to a crystalline structure.
Thin film formation: The processing of thin films, such as spin coating or vapor deposition, often results in abrupt solidification, causing the polymer chains to freeze in a disordered configuration.
Solvent interactions: The choice of solvent during the film deposition process may potentially influence crystallization. Extremely rapid evaporation or inadequate dissolution of PEDOT by solvents often leads to the formation of amorphous films.
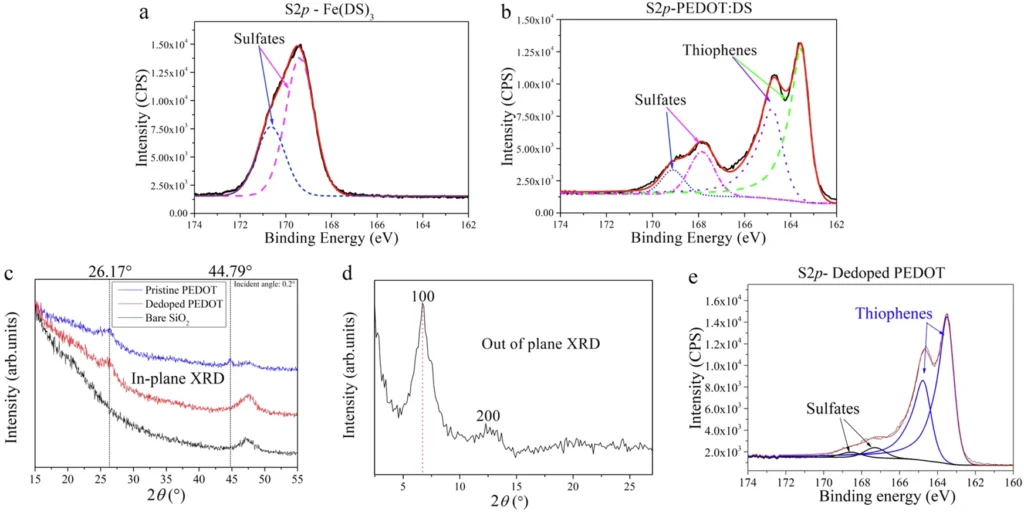
Researchers have employed numerous techniques to manipulate the crystallization process of PEDOT, including thermal annealing, solvent vapor annealing, and chemical templating. Nevertheless, these methodologies frequently yield incongruous outcomes or pose challenges in terms of scalability. As a result, there has been an increasing fascination with the use of self-assembling surfactants such as iron(III) dodecyl sulfate, which can enhance the crystallization process’s efficiency. XPS and XRD spectra of Fe(DS)3 and PEDOT:DS films.
What is the definition of directed crystallization?
Directed crystallization is the deliberate imposition of an external force or template to direct the formation of crystals in a certain orientation or pattern. Unlike spontaneous crystallization, which arises spontaneously but inherently generates random or disordered patterns, directed crystallization enables greater manipulation and accuracy in determining the final crystal structure.
In polymers such as PEDOT, directed crystallization is particularly advantageous since it effectively counteracts the inherent inclination of the polymer chains to maintain a state of disorder. The provision of a template or guide can effectively stimulate the alignment of polymer chains, thereby enhancing crystallinity and resulting in enhanced electrical and mechanical characteristics.
You can use a variety of methods to direct the crystallization process, including:
Thermal gradients are a phenomenon in which temperature variations within a material promote crystal growth in specific orientations.
Magnetic or electric fields can facilitate the alignment of specific types of molecules.
Chemical templates are pre-existing structures that promote crystal development in a specific orientation.
The most effective method for PEDOT preparation is using chemical surfactants, specifically iron(III) dodecyl sulfate.
Iron(III) Dodecyl Sulfate plays a crucial role in the industrial process:
Iron(III) dodecyl sulfate is a surfactant that is made up of iron(III) ions (Fe2) and dodecyl sulfate molecules (C₁₃H₀₈SO₄2). The dodecyl sulfate component possesses an elongated hydrophobic tail, whereas the iron(III) ion exhibits hydrophilicity. Under certain circumstances, its amphiphilic character enables it to spontaneously aggregate into organized formations, particularly lamellar superstructures.
Within the framework of PEDOT crystallization, iron(III) dodecyl sulfate fulfills two main functions:
Chemical dopants such as iron(III) ions, which act as oxidizing agents, promote the polymerization of EDOT (the monomer of PEDOT) into its conducting form, PEDOT.
Structural guide: The dodecyl sulfate molecules undergo self-assembly to form lamellar frameworks, which serve as a template for the crystallization of PEDOT.
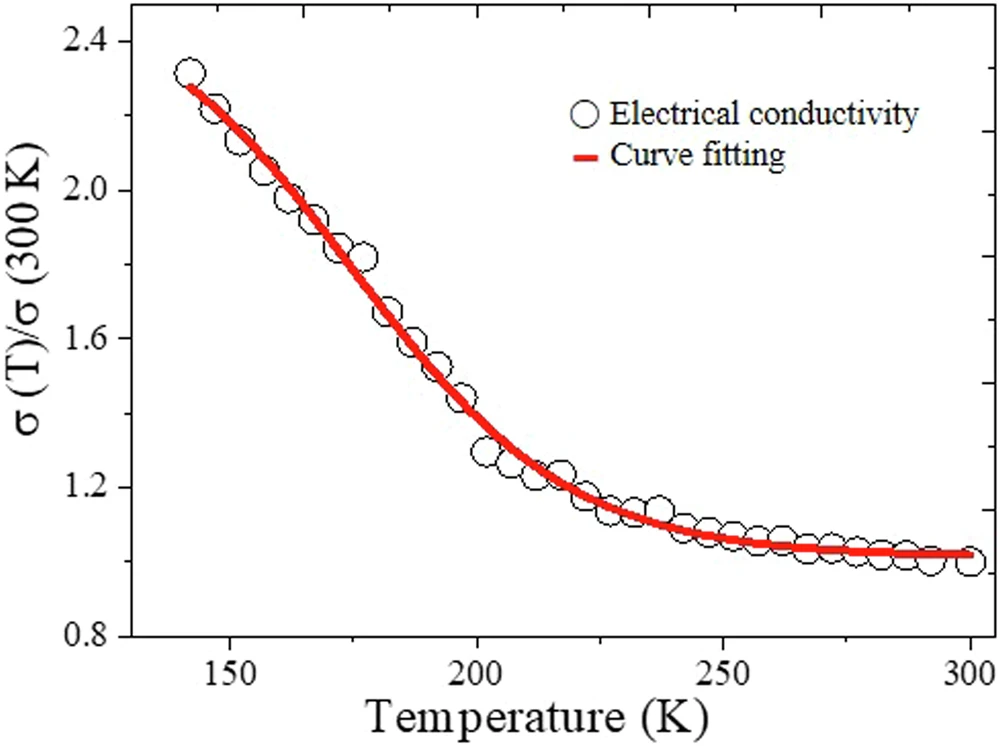
Iron(III) dodecyl sulfate, which provides both a chemical and physical guide, facilitates PEDOT crystallization, resulting in more consistent films with increased crystallinity. Temperature dependence of conductivity.
A Comprehensive Analysis of Lamellar Superstructures:
A lamellar superstructure is a structure composed of parallel layers or sheets of material. They are a common occurrence in the field of materials science, specifically in the study of liquid crystals and biological membranes. Surfactant molecules self-organize in Iron(III) dodecyl sulfate into lamellar phases, forming a structured and repetitive arrangement that serves as a template for crystallogram formation.
During the crystallization process, the lamellar structure offers several advantages:
Guided alignment: Through guided alignment, the lamellar structure directs PEDOT chains to align more organized when they undergo polymerization.
Reduction of defects: An ordered structure in the film helps minimize flaws, resulting in improved mechanical and electrical properties.
Scalability: The spontaneous formation of the lamellar structure makes this method extremely easily scalable, which is crucial for industrial applications.
Molecular interactions between PEDOT and Iron(III) Dodecyl Sulfate:
The guided crystallization process is crucially dependent on the interaction between PEDOT and iron(III) dodecyl sulfate. This interaction manifests at both the chemical and physical levels.
Chemically, In a metabolic sense, the iron(III) ions work as oxidizing agents that make it easier for EDOT to polymerize into PEDOT. Hence, the iron(III) dodecyl sulfate functions as both a structural template and an active contributor to the polymer’s synthesis. Such dual functionality makes it particularly efficient for targeted crystallization.
Physically, The surfactant’s dodecyl sulfate component adopts a lamellar lattice structure. This structure serves as a framework that directs the development of PEDOT crystals. The polymer chains proliferate along the layers, resulting in a more structured morphology than would arise spontaneously.
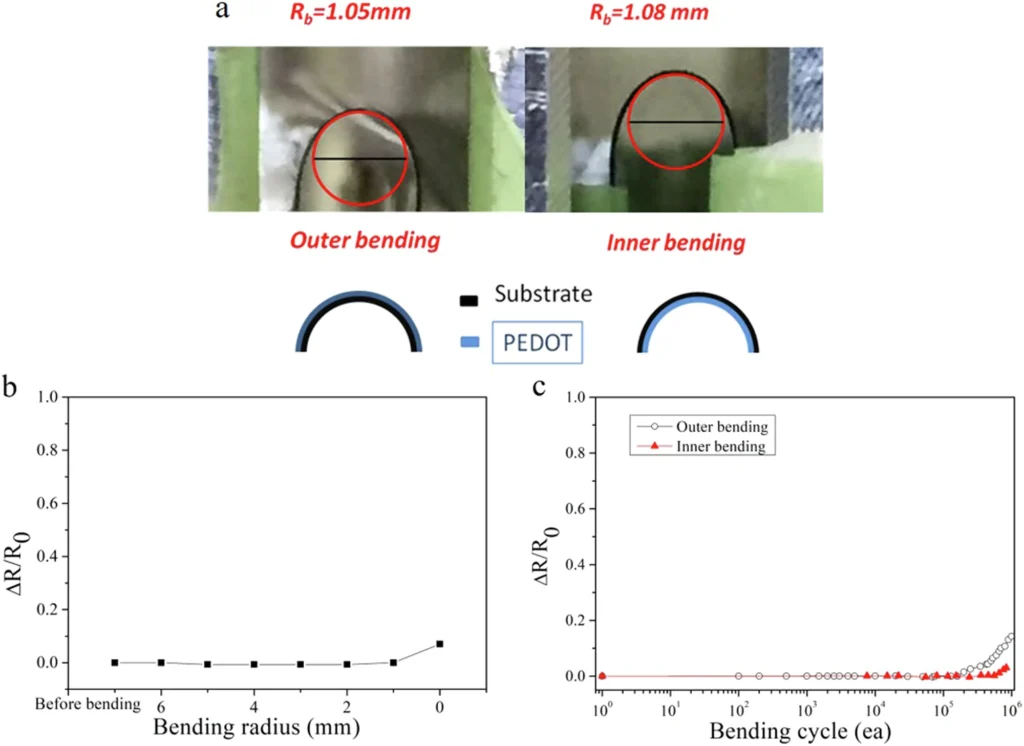
Because of the two interactions mentioned above, iron(III) dodecyl sulfate can effectively control the crystallization process. This creates films that are both very crystalline and very conductive. Mechanical durability test of PEDOT/PET film.
Investigation of the Directed Crystallization Mechanism in PEDOT Films:
Guided crystallization of PEDOT films using iron(III) dodecyl sulfate is a process that has several steps that happen in a certain order. Understanding this mechanism can provide a valuable understanding of the reasons behind this strategy’s superior effectiveness in comparison to alternative methods of crystallizing PEDOT.
Self-Assembly of Iron(III) Dodecyl Sulfate: Self-assembly initiates the process by which iron(III) dodecyl sulfate forms a lamellar structure. When hydrophilic iron(III) ions and hydrophobic dodecyl sulfate tails arrange themselves into layers, a highly ordered template is formed.
Polymerization of EDOT: Iron(III) ions act as oxidizing agents in EDOT polymerization, promoting the formation of PEDOT crystals. Upon polymerization, the PEDOT chains initiate growth along the lamellar structure.
Alignment of PEDOT Chains: The lamellar structure governs the evolution of PEDOT chains, directing them to align more systematically. Consequently, this results in a higher level of crystallinity compared to the case where the polymer crystallizes naturally.
Formation of Crystalline Domains: The assembly of PEDOT chains in a systematic manner results in the creation of crystalline domains inside the film. Polymer domains are areas characterized by the effective alignment of polymer chains for efficient charge transport.
The outcome is a PEDOT film characterized by enhanced crystallinity, reduced flaws, and increased electrical conductivity.
Examining the morphological consequences of directed crystallization:
Iron(III) dodecyl sulfate causes PEDOT to crystallize in a specific way, which has a big effect on the shape of the film that is being made. Specifically, it produces a film that exhibits the following characteristics:
Enhancement of crystallinity: The lamellar structure encourages the formation of crystalline domains, which are regions of the film with tightly arranged polymer chains.
Defect reduction: The guided crystallization method effectively minimizes the presence of defects in the film, such as disordered areas or voids, which can have a detrimental effect on the film’s performance.
Enhanced uniformity: The spontaneous formation of the lamellar structure results in a more homogeneous film, which is crucial for applications that need constant performance over extensive regions.
These morphological enhancements are particularly useful for applications like organic electronics, where the film’s operational efficiency is closely linked to its structure.
Enhancements in Conductivity Resulting from Crystallization:
One of the primary benefits of targeted crystallization in PEDOT films is increased electrical conductivity. The relationship between crystallinity and conductivity is well established: an increase in crystallinity results in enhanced mobility of charge carriers (electrons or holes), thereby positively affecting the overall conductivity.
A PEDOT film with a high degree of crystallinity exhibits improved alignment of polymer chains, therefore facilitating more effective routes for charge transfer. This is especially significant for applications such as organic field-effect transistors (OFETs), supercapacitors, and solar cells, where optimal device performance relies on strong conductivity.
Researchers have found that PEDOT films made by crystallizing iron(III) dodecyl sulfate are much more conductive than films made using other methods. Such characteristics render the approach especially appealing for high-performance electrical devices. Stretchability and water-stability test of PEDOT:DS film.
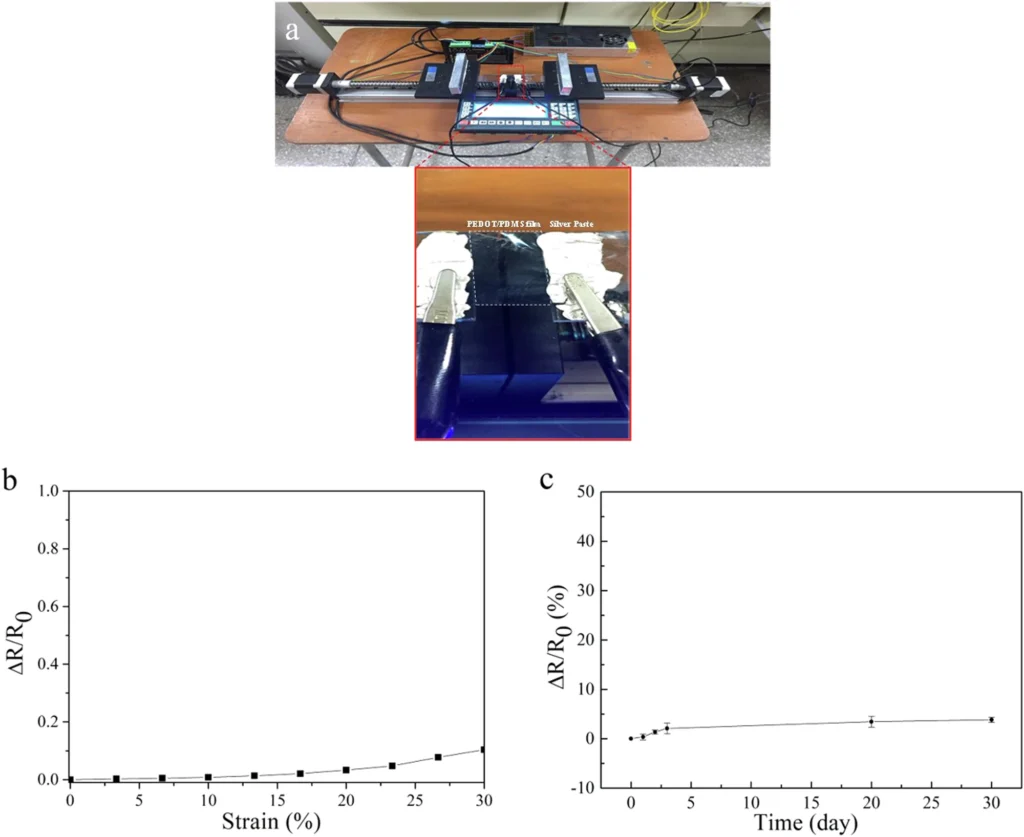
Comparative Analysis of Alternative Crystallization Methods:
There are several other techniques available to promote crystallization in PEDOT films, including:
Thermal annealing: Thermal annealing is the process of heating the film to a precise temperature to promote crystallization.
Solvent vapor annealing: The process of solvent vapor annealing involves subjecting the film to solvent vapors, which stimulate the polymer chains to undergo reorganization and adopt a more structured arrangement.
Vapor-phase polymerization: The technique of vapor-phase polymerization involves polymerizing the EDOT monomer in the vapor phase, leading to the creation of stronger crystalline sheets.
Each of these approaches has its own merits and drawbacks. For instance, thermal annealing is a somewhat straightforward process yet frequently yields incongruous end products. Solvent vapor annealing is capable of generating films with intricate crystal structures, but it has challenges in terms of control and scalability. Despite its complexity and high cost, vapor-phase polymerization can yield satisfactory outcomes.
In contrast, the use of iron(III) dodecyl sulfate is notable due to its innovative combination of chemical and physical guidance in the crystallization process. In comparison to other methodologies, the use of this dual technique results in films that exhibit greater crystallinity, enhanced conductivity, and superior mechanical characteristics.
Utilizations of Crystallized Polyether Dioxy (PEDOT) Films:
Guided crystallization with iron(III) dodecyl sulfate improves the crystallinity and conductivity of PEDOT films, opening up new uses for them in several situations. Several highly promising areas include:
Organic Electronics: The use of crystalline PEDOT films as the active layer in organic transistors results in enhanced conductivity, allowing for faster switching rates and increased device efficiency.
Supercapacitors: The superior conductivity of crystalline PEDOT films is responsible for the enhanced charge storage capacity and accelerated charging/discharging periods in energy storage devices like supercapacitors.
Solar Cells: The use of crystalline PEDOT films as the hole transport layer in organic solar cells has been shown to improve charge extraction efficiency and, consequently, the cell’s overall power conversion efficiency.
Flexible Electronics: These crystalline PEDOT films have better mechanical properties, which makes them ideal for use in flexible and stretchable electronics that need the best mix of high conductivity and mechanical strength.
The methodology’s drawbacks and limitations:
While there are some advantages to using iron(III) dodecyl sulfate for targeted crystallization in PEDOT films, there are also some challenges and limitations to consider:
Scalability: Although the method achieves satisfactory results in small-scale trials conducted in a laboratory, it may provide difficulties when expanded for industrial use. The lamellar structure’s self-assembly depends on specific circumstances, which can be difficult to replicate on a larger scale with consistency.
Residual Surfactants: Post-crystallization, leftover surfactant molecules may persist in the film, potentially affecting its performance detrimentally. Further investigation is required to devise techniques for eliminating or reducing these residuals.
Process Optimization: Directed crystallization using iron(III) dodecyl sulfate is a recently developed technique, and there is still much to learn about the optimization of variables like surfactant concentration and polymerization temperature for various applications.
Possible paths for additional research and progress:
The continuous development in the field of PEDOT crystallization presents numerous promising opportunities for future research. Some possible avenues of exploration include:
Investigation of New Surfactants: While iron(III) dodecyl sulfate has been shown to work, other surfactants may have similar or even better results. Researchers are currently investigating certain self-assembling compounds that could potentially direct crystallization in novel ways.
Integration with Flexible Electronics: The growing demand for flexible and wearable electronics has sparked considerable interest in the development of stretchable PEDOT films that exhibit excellent conductivity. The implementation of directed crystallization techniques has the potential to significantly enhance the durability and efficiency of these films.
Hybrid Materials: Integration of PEDOT with other materials, such as graphene or carbon nanotubes, has the potential to significantly augment its characteristics. Direct crystallisation may facilitate the formation of well-structured hybrid materials with combined characteristics.
Summary:
Using iron(III) dodecyl sulfate to control the crystallization of PEDOT films is a new method that greatly improves the electrical conductivity and structural properties of this important conducting polymer. This approach effectively addresses the difficulties associated with crystallizing PEDOT by manipulating the crystallization process by inducing the development of a lamellar superstructure, resulting in films that exhibit enhanced performance and increased adaptability for various applications. Despite the existing obstacles, namely scalability and process optimization, the prospects for this approach appear promising, and it signifies a significant advancement in the field of high-performance conducting polymers.
Frequently Asked Questions:
1). What is the primary benefit of utilizing iron(III) dodecyl sulfate in PEDOT crystallization?
The compound iron(III) dodecyl sulfate serves as both a dopant and a structural template, facilitating PEDOT crystallization and enhancing its crystallinity and conductivity.
2). To what extent does direct crystallization enhance the performance of PEDOT films?
Directional crystallization makes it easier for PEDOT chains to line up in a planned way, which improves their electrical conductivity and mechanical properties.
3). Describe lamellar superstructures and explain their significance.
Ordered, layered structures, known as lamellar superstructures, direct the development of polymer chains during crystallization, resulting in more homogeneous and crystalline films.
4). Is this crystallization technique applicable to other polymers?
While PEDOT has been the focus of this technology’s optimization, other conducting polymers can benefit from a similar methodology using self-assembling surfactants.
5). What are the primary obstacles to expanding this approach for industrial applications?
The main obstacles include ensuring uniform self-assembly of the lamellar structure on a larger scale and resolving any problems with remaining surfactants in the final film.
For more chemistry blogs, visit chemistry Master