Table of Contents
Fundamentals of Electrochemical Sensing of Vitamin B6:
Electrochemical sensing represents a highly promising technology in contemporary analytical chemistry. The process entails quantifying electrical signals generated by the interaction between a target molecule, such as a vitamin or ion, and a specific sensor. In recent decades, electrochemical sensors have gained prominence due to their rapid response, high sensitivity, and affordability. Diverse sectors, from healthcare to environmental monitoring, utilise them for real-time data acquisition and accurate measurements.
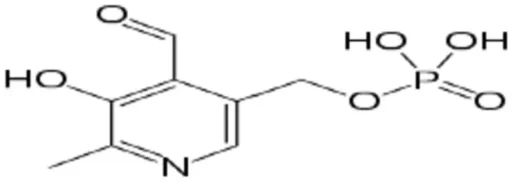
A primary benefit of electrochemical sensing is its adaptability. Altering the electrode surface enables sensors to be customized to identify specific analytes, ranging from basic ions to intricate chemical compounds such as vitamins. Vitamin B6, or pyridoxine, is increasingly garnering attention in this domain. Accurate detection and quantification of Vitamin B6 is crucial in food analysis, pharmaceutical quality control, and clinical diagnostics. Scheme 1 Vitamin B6.
The importance and role of vitamin B6 in human health is significant:
Vitamin B6, comprising several vitamers such as pyridoxine, pyridoxal, and pyridoxamine, is essential for more than 100 enzymatic reactions in the human body. It is crucial for protein metabolism, neurotransmitter production, and the regulation of homocysteine levels, an indicator of cardiovascular disease risk. Additionally, Vitamin B6 has a role in immunological function, haemoglobin production, and the synthesis of neurotransmitters like serotonin, dopamine, and gamma-aminobutyric acid (GABA).
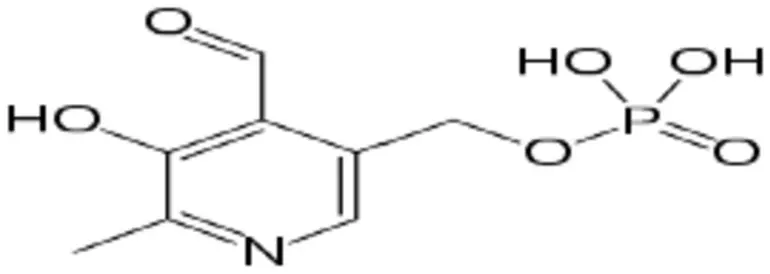
A deficit in Vitamin B6 may result in various health complications, such as anaemia, dermatological conditions, depression, and compromised immune function. As a result, there is a growing demand for dependable and sensitive techniques for detecting Vitamin B6, both in biological specimens and in fortified foods and pharmaceutical items. Scheme 2
An Overview of Electrochemical Sensors:
Electrochemical sensors operate by identifying chemical processes at the electrode surface, transforming chemical data into a quantifiable electrical signal. The primary components of an electrochemical sensor are the working electrode, where the reaction transpires, and the reference electrode, which supplies a constant voltage.
Various categories of electrochemical sensors exist, including:
Amperometric sensors quantify the current generated by an electrochemical process under a constant voltage.
Potentiometric sensors assess the voltage differential between two electrodes.
Conductometric Sensors: These measure variations in a solution’s conductivity as a result of a reaction.
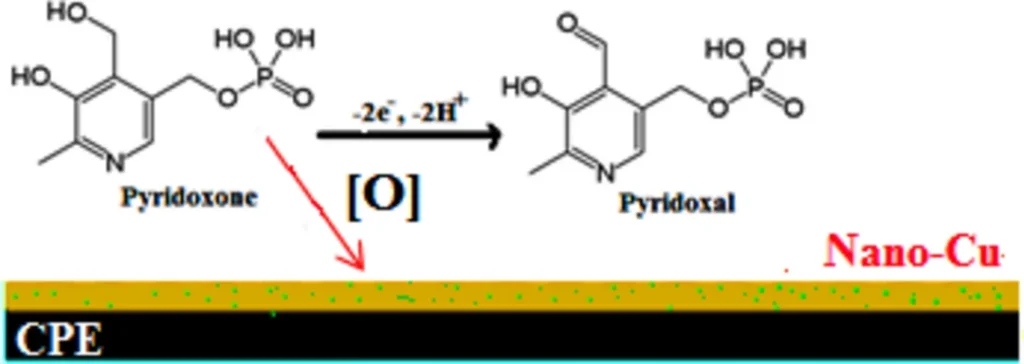
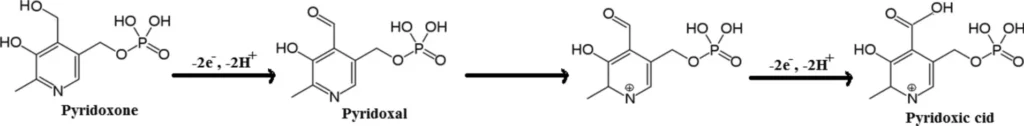
Amperometric sensors, particularly those that use carbon-based electrodes, find extensive use in the detection of vitamins like Vitamin B6, due to their sensitivity and versatility. These applications favour carbon paste electrodes (CPEs) due to their cost-effectiveness, ease of preparation, and the ability to replace them with other materials to enhance performance. Scheme 3 The suggested oxidation mechanism:
Carbon paste electrodes: composition and operation
Carbon paste electrodes are composite electrodes created by amalgamating carbon powder with a binder, usually an organic liquid such as oil or paraffin. This amalgamation creates a paste that easily fills a hollow or coats a surface, forming an electrode. The straightforward architecture and adjustable characteristics of the paste render CPEs suitable for various applications, especially in electrochemical sensing.
The ability to enhance CPEs with various materials such as nanoparticles, polymers, or catalysts enhances their sensitivity and selectivity for specific analytes. These alterations can augment the electrode’s surface area, enhance its conductivity, and perhaps provide particular binding sites for the target molecule. Modifying the CPE for Vitamin B6 detection can substantially enhance its efficacy.
What is the rationale for using carbon paste electrodes to detect Vitamin B6?
The identification of vitamins, particularly in intricate matrices such as food or biological specimens, necessitates sensors that are both sensitive and selective. In this context, carbon paste electrodes have numerous advantages:
Customizable Surface: You can easily modify CPEs with different materials to boost their effectiveness for specific applications. To find vitamin B6, nanoparticles may be used to increase the surface area and make electron transfer easier, or enzymes with specific binding sites for pyridoxine may be used.
Economical: Carbon paste electrodes are comparatively low-cost to manufacture in relation to other electrode types, rendering them a compelling choice for extensive or commercial uses.
Simplicity of Fabrication: The ease of CPE fabrication enables researchers to experiment with various compositions and changes quickly. This adaptability makes them ideal for electrochemical sensing research and development.
Wide Potential Range: CPEs have a wide potential range, allowing for the detection of several analytes, including vitamins like pyridoxine.
The characteristics of CPEs make them an exceptional option for electrochemical detection of Vitamin B6, particularly when enhanced by changes that increase their sensitivity and selectivity.
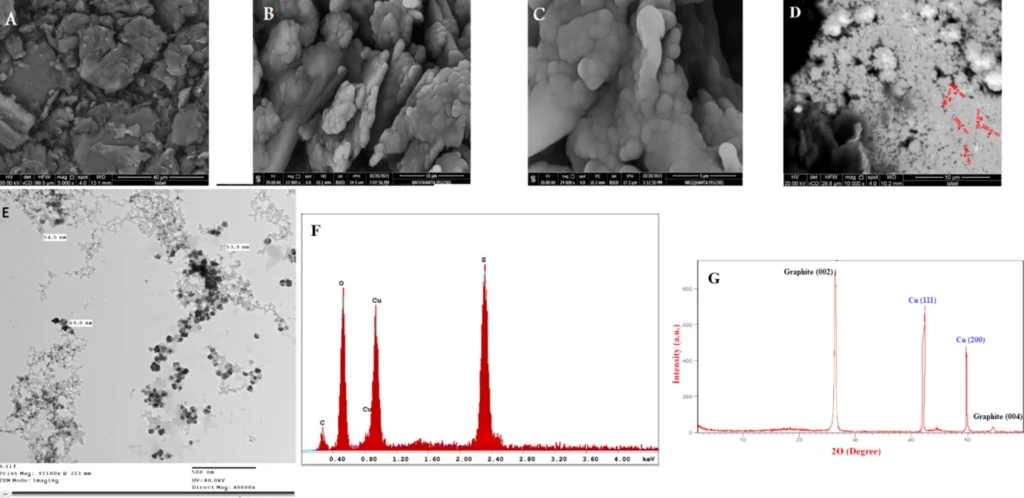
SEM images of (A) CPE and (B–D) CuNCPE with different magnifications. (E) Transmission electron microscopy (TEM) micrograph of CuNs (F) EDX spectrum of CuNs on CPE and (G) XRD pattern of CuNCPE.
Electrochemical Detection Mechanism of Vitamin B6:
Several small organic compounds, including pyridoxine, can be detected electrochemically by redox reactions. These are reactions in which the target molecule either takes on or gives up electrons at the electrode surface. When a pyridoxine molecule comes into contact with the surface of a carbon paste electrode, it starts a chain of electron transfer reactions that create a measurable current.
In most electrochemical sensors, the magnitude of this current is precisely proportional to the concentration of the analyte, specifically pyridoxine, in the solution. By quantifying the current produced at various potentials, researchers can ascertain both the quantity of pyridoxine and its distinct electrochemical characteristics.
Enhancement of Carbon Paste Electrodes for Improved Performance:
Modification can significantly improve the efficacy of carbon paste electrodes for electrochemical sensing, despite their inherent value. Numerous approaches exist to enhance CPEs for pyridoxine detection, primarily nanomaterials or catalysts that increase the electrode’s sensitivity, selectivity, and stability.
Nanoparticles: Incorporating nanoparticles into carbon paste significantly enhances the electrode’s surface area, hence increasing the availability of active sites for electrochemical reactions. The enlarged surface area facilitates enhanced electron transport, hence improving the sensor’s sensitivity. In this context, gold, silver, and carbon nanotubes are frequently used nanoparticles.
Conductive Polymers: A common change is the incorporation of conductive polymers, which improve the electrode’s electrical conductivity. These polymers can additionally incorporate certain functional groups that interact with pyridoxine, thereby enhancing the sensor’s selectivity.
Catalysts: Certain modifications entail the incorporation of catalytic substances that can reduce the activation energy required for the redox reaction of pyridoxine, thereby enhancing the detection process’s efficiency. Catalysts may consist of metals, metal oxides, or other catalytic substances.
By integrating these elements into the carbon paste, researchers may develop electrodes that are highly sensitive and selective, capable of detecting tiny quantities of Vitamin B6 in intricate samples.
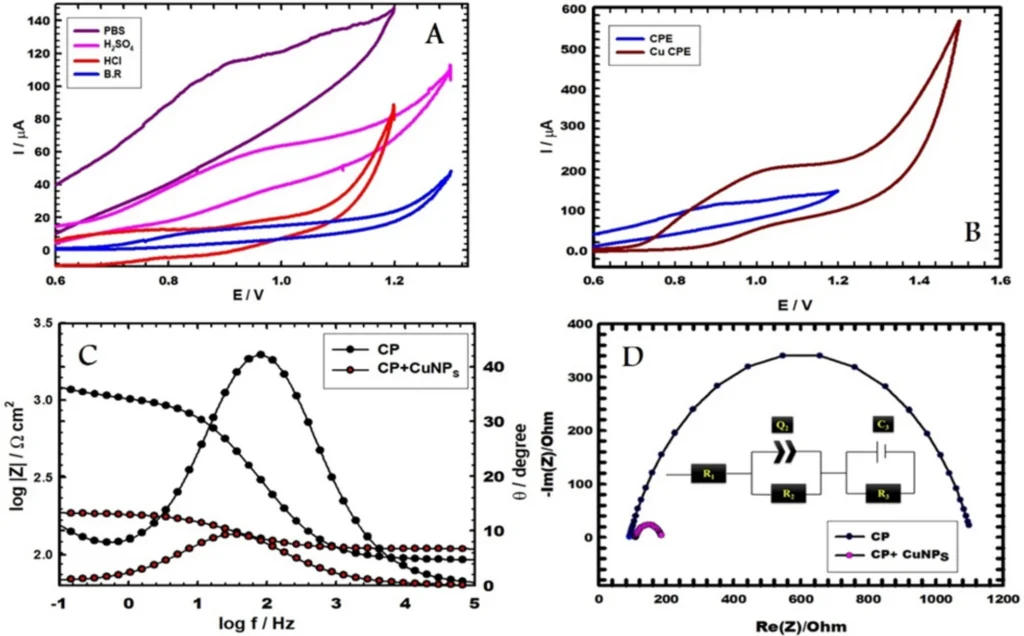
photo credit (A) CVs of bare CPE utilizing different supporting electrolytes with 1.0 mM vitamin B6 at scan rate 0.05 V s−1. (B) CVs of CPE and CuNCPE (in PBS, pH 5.0) with 1.0 mM vitamin B6. (C, D) Bode and Nyquist plots of CPE and CuNCPE (in PBS, pH 5.0) with 1.0 mM vitamin B6 (D inset: equivalent circuit).
Electrochemical Methods for Pyridoxine Detection:
We employ various electrochemical methods to identify pyridoxine, each with distinct advantages and uses. The primary methods used for detecting vitamin B6 include:
Cyclic Voltammetry (CV): Cyclic Voltammetry (CV) is a widely used electrochemical technique that produces a cyclic voltammogram by linearly varying the potential of the working electrode over time. This method is especially advantageous for examining the redox properties of pyridoxine, as it offers comprehensive insights into the oxidation and reduction reactions occurring at the electrode interface.
Differential Pulse Voltammetry (DPV): Differential pulse voltammetry (DPV) is a very sensitive method that employs a sequence of voltage pulses at the working electrode to measure the resultant current. This method is better than cyclic voltammetry at telling the difference between a number of electrochemical phenomena, making it ideal for finding low levels of pyridoxine.
Square Wave Voltammetry (SWV): Square Wave Voltammetry (SWV) is a rapid and extremely sensitive method that utilises a square wave potential at the electrode. This method is especially adept at identifying trace levels of pyridoxine in intricate materials, rendering it a favoured option for food and pharmaceutical analysis applications.
Each technique has distinct advantages and disadvantages, and the choice of a technique is contingent upon the experiment’s unique demands, including required sensitivity, sample complexity, and desired analysis time.
Enhancing Experimental Parameters for Vitamin B6 Detection:
The experimental conditions in which electrochemical sensors, like carbon paste electrodes, operate can significantly impact their efficacy. We must improve numerous critical aspects to provide precise and trustworthy findings in pyridoxine detection.
pH: The pH of the solution significantly influences pyridoxine’s electrochemical behavior. The redox potentials of pyridoxine may vary at different pH levels, influencing the current response. Consequently, optimising the pH of the solution is essential for achieving the most precise and reproducible findings.
Temperature influences both the electrochemical reaction’s kinetics and the electrode’s stability. Elevated temperatures may enhance the reaction rate; however, they can also result in increased signal noise. In contrast, reduced temperatures can enhance the stability of the electrode, although they may impede the reaction rate.
Scan Rate: In cyclic voltammetry, the scan rate indicates the speed at which the potential changes. The scan rate significantly influences the morphology and amplitude of the cyclic voltammogram, necessitating meticulous control to guarantee precise results.
Electrolyte Concentration: The concentration of the electrolyte in the solution can influence the sensor’s performance. An increased electrolyte content may enhance the solution’s conductivity, resulting in a more robust current response. However, it may also increase background noise, complicating the detection of the analyte.
Through meticulous optimisation of these and more experimental parameters, researchers can enhance sensor performance and guarantee the provision of precise and dependable data.
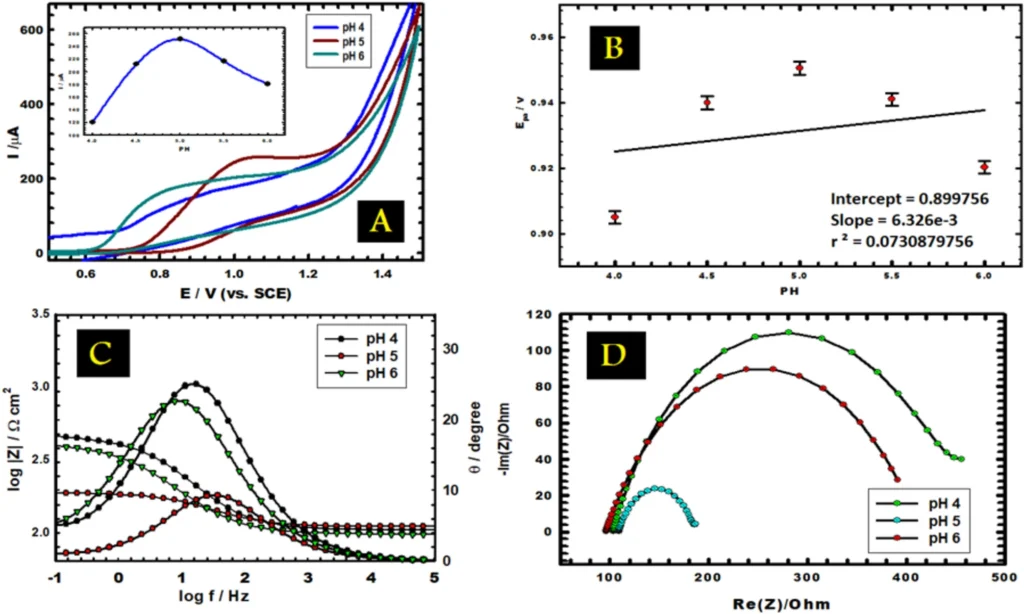
photo credit (A) CVs of vitamin B6 in 0.1 M PBS at different pH (4.0–6.0) at scan rate 0.05 V s−1. Inset, the variation of anodic peak current with pH. (B) The effect of pH on the anodic peak potential (C, D) Bode and Nyquist plots of 1.0 mM vitamin B6 at different pH values.
Carbon Paste Electrodes’ Essential Performance Indicators:
When evaluating the effectiveness of carbon paste electrodes for Vitamin B6 detection, we must consider several critical criteria:
Sensitivity: Sensitivity denotes the sensor’s capacity to identify minor variations in pyridoxine content. A highly sensitive sensor can detect lower analyte concentrations, which is critical in situations where pyridoxine is present in trace quantities.
The detection limit refers to the minimum concentration of pyridoxine that the sensor can accurately identify. This measure is crucial in applications such as clinical diagnostics, where detecting minimal concentrations of pyridoxine in biological samples may be essential.
Selectivity denotes the sensor’s capacity to precisely identify pyridoxine among other analogous chemicals. This is especially crucial in intricate samples like food or biological fluids, where additional vitamins, ions, or chemical compounds may exist.
Reproducibility denotes the sensor’s capacity to yield consistent outcomes across numerous measurements. Elevated repeatability is crucial for guaranteeing the sensor delivers dependable data.
Stability denotes the sensor’s capacity to sustain its performance over time. Sensors that deteriorate or diminish in sensitivity with prolonged use are unsuitable for practical applications.
By enhancing these performance criteria, researchers can guarantee that their carbon paste electrodes deliver precise, dependable, and sensitive detection of pyridoxine.
Selectivity of Adapted Carbon Paste Electrodes:
A primary problem in electrochemical sensing is guaranteeing the sensor’s selectivity for the target analyte, specifically Vitamin B6. Several other substances, such as food, medications, or biological fluids, frequently exist in intricate samples that may disrupt the detection process. Other vitamins, amino acids, or ions may generate analogous electrochemical signals, complicating the precise quantification of pyridoxine.
Researchers have devised multiple techniques to improve the selectivity of carbon paste electrodes for pyridoxine detection.
Surface Modification: An effective method to improve a sensor’s selectivity is to alter the electrode’s surface with compounds that are particularly pyridoxine-sensitive. You can fix enzymes or antibodies on the electrode’s surface to create precise binding sites for pyridoxine and minimise interference from other substances.
The incorporation of nanomaterials, such as gold nanoparticles and carbon nanotubes, into the carbon paste can enhance its selectivity. These materials can augment the electrode’s surface area and offer unique binding sites for pyridoxine, hence enhancing the sensor’s capacity to differentiate it from other molecules.
Electrochemical Pretreatment: In some cases, treating the electrode with a designated potential or chemical solution may improve its pyridoxine selectivity. This procedure can aid in eliminating undesirable impurities from the electrode’s surface or augment its interaction with pyridoxine.
By implementing these methodologies, researchers can develop sensors that exhibit outstanding selectivity for pyridoxine, even in intricate samples.
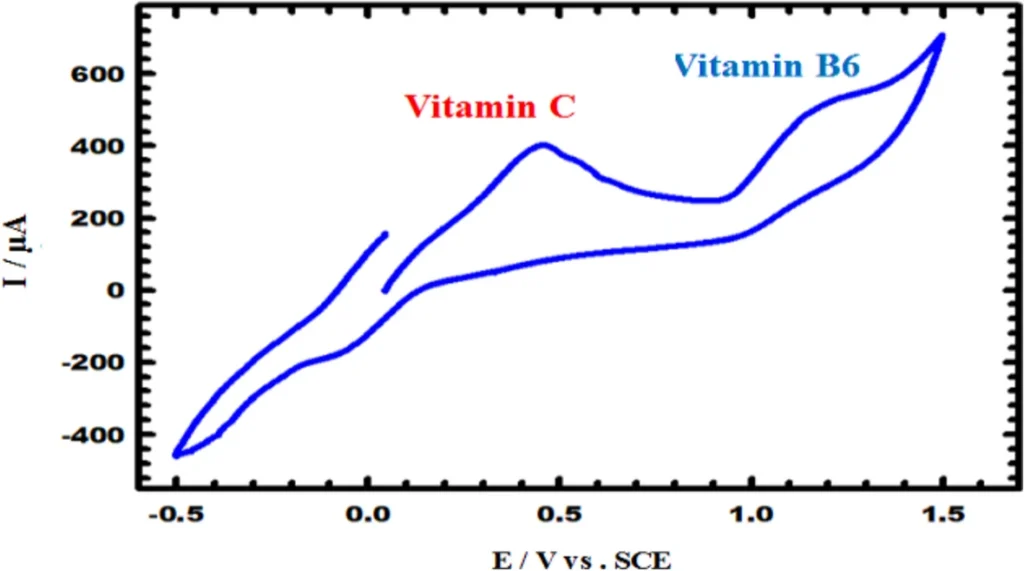
CV for the selectivity of CuNCPE to 5 × 10–4 M vitamin B6 in the existence of Ascorbic acid in 0.1 M PBS (pH 5.0) with scan rate 0.05 Vs−1.
Electrochemical pyridoxine detection is utilized:
The precise and dependable detection of pyridoxine holds significant significance for various industries, including food processing, medicines, and healthcare.
Food Industry: In the food sector, precise identification of pyridoxine is crucial for verifying that fortified goods have the appropriate levels of Vitamin B6. Electrochemical sensors offer a rapid, economical approach for assessing vitamin concentrations in food items, ensuring adherence to regulatory requirements, and delivering precise nutritional data to customers.
Pharmaceutical Industry: The pharmaceutical business frequently uses pyridoxine in supplements and pharmaceuticals. Electrochemical sensors can monitor the quality and consistency of these products, ensuring they contain the appropriate quantity of Vitamin B6.
Clinical Applications: In healthcare, the precise detection of pyridoxine in biological samples is crucial for the diagnosis and management of vitamin B6 insufficiency. Point-of-care testing devices employ electrochemical sensors to rapidly and precisely quantify pyridoxine levels in blood or urine samples, providing clinicians with essential data for diagnosing and managing deficits.
Obstacles and limitations in pyridoxine detection:
Despite the many advantages of electrochemical sensors, there are still some challenges and limitations to overcome.
Interference from Other Compounds: The presence of other compounds in the sample causes significant interference in pyridoxine detection. Several vitamins, ions, or organic compounds may produce similar electrochemical signals in complex mixtures like food or biological fluids. This makes it harder to precisely measure the amount of pyridoxine present.
Stability of Modified Electrodes: The stability of new electrodes over time is another challenge. Modifications may enhance electrode performance but can also increase its vulnerability to degradation, especially in extreme conditions. Researchers must devise techniques to guarantee the stability and reliability of modified electrodes during extended periods of utilisation.
Detection of Pyridoxine in Complex Matrices: Identifying pyridoxine in intricate matrices such as food or biological fluids poses challenges due to the interference of other substances. Researchers must devise techniques to enhance the selectivity and sensitivity of their sensors in these intricate contexts.
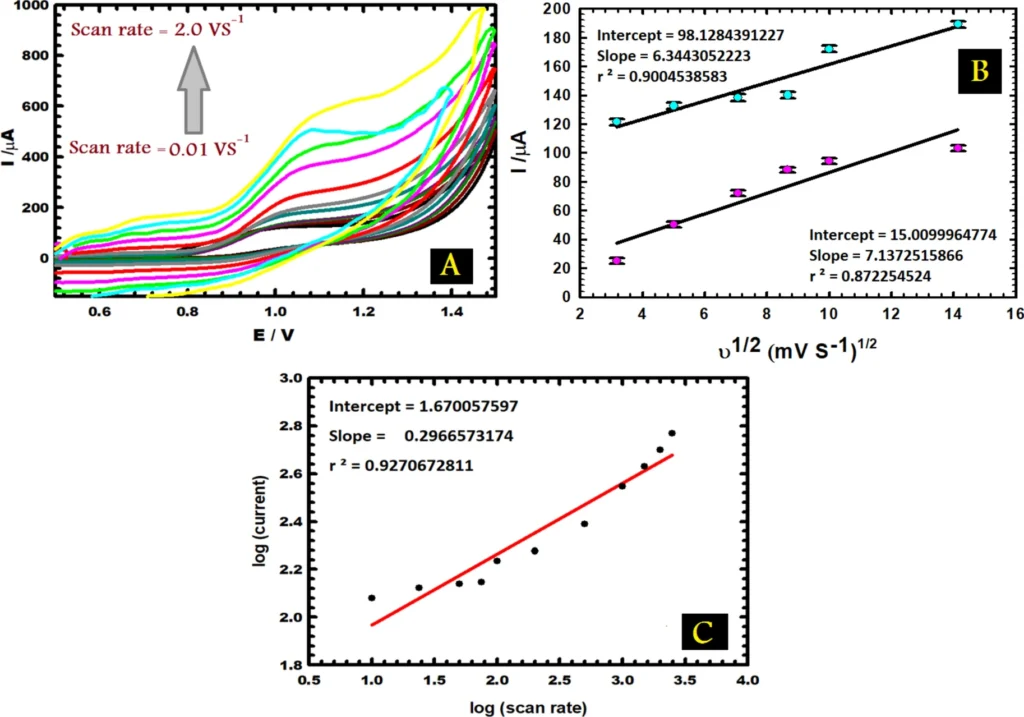
photo credit A) CVs of 1.0 mM vitamin B6 (in PBS, pH 5.0) with different scan rates (0.01–2.0 V s−1). (B) The variation of the anodic peak current with the square root of scan rate (C) The variation of the logarithm of the anodic peak current with the logarithm of the scan rate.
Recent developments in carbon paste electrodes for Vitamin B6 detection:
Recent breakthroughs in nanotechnology and materials science have resulted in notable enhancements in the efficacy of carbon paste electrodes for the detection of Vitamin B6. Notable advancements encompass:
Nanomaterials: The integration of nanomaterials, including gold nanoparticles, silver nanoparticles, and carbon nanotubes, into carbon paste electrodes has significantly enhanced their sensitivity and selectivity. These materials offer an expanded surface area for the electrochemical reaction, enhancing electron transport and augmenting the sensor’s overall efficiency.
Conductive Polymers: The use of conductive polymers, including polyaniline and polypyrrole, has enhanced the efficacy of carbon paste electrodes. These polymers can enhance electrode conductivity and offer particular binding sites for pyridoxine, hence improving the sensor’s sensitivity and selectivity.
New Materials: Researchers are looking into how to improve the performance of carbon paste electrodes by using new materials like graphene and metal-organic frameworks (MOFs). These materials provide distinctive characteristics that can improve the sensitivity, selectivity, and stability of the sensor.
Future Prospects and Emerging Innovations in Electrochemical Vitamin Sensing:
Portable and Wearable Devices: The integration of carbon-paste electrodes into portable and wearable electronics represents a significant advancement. These technologies may provide continuous real-time monitoring of vitamin levels, offering significant insights for healthcare, nutrition, and fitness applications.
Biosensing Platforms: Another rising trend is the development of biosensing platforms that use carbon paste electrodes. These platforms could improve the accuracy of finding pyridoxine by adding biological recognition elements, like enzymes or antibodies, to the sensor.
Future Research Directions: In the future, scientists will probably focus on making carbon paste electrodes more sensitive, selective, and stable, as well as coming up with new materials and ways to make them work better. There is considerable potential for the advancement of multi-analyte sensors capable of simultaneously detecting various vitamins, offering a more thorough assessment of nutritional status.
Final Assessment:
The electrochemical detection of Vitamin B6 via modified carbon paste electrodes provides an effective and adaptable method for vitamin sensing. Researchers can substantially enhance the sensitivity and selectivity of sensors by altering the electrode surface with nanomaterials, conductive polymers, and other substances, rendering them suitable for use in food analysis, pharmaceuticals, and healthcare. Despite existing limitations, recent progress in materials science and nanotechnology offers significant potential for the future of electrochemical vitamin sensing. We anticipate that ongoing research will yield increasingly inventive solutions that enhance the accuracy, reliability, and accessibility of these sensors.
Frequently Asked Questions:
1). What are the benefits of carbon paste electrodes?
Carbon paste electrodes have many advantages, such as being cheap, easy to make, and easily able to be changed by adding nanomaterials, polymers, or catalysts to make them work better.
2). What is the electrochemical sensing mechanism for Vitamin B6?
Electrochemical sensing works by measuring the current that flows when pyridoxine undergoes a redox reaction on the surface of the electrode. The current correlates with the concentration of pyridoxine in the sample, facilitating its quantification.
3). Are CPEs capable of detecting more vitamins?
Indeed, by modifying the surface chemistry of the electrode to selectively target certain analytes, we can adapt carbon paste electrodes to identify different vitamins, such as Vitamin C or Vitamin E.
4). What are the prevalent interferences in electrochemical sensing?
Other vitamins, ions, and organic compounds within the sample may generate analogous electrochemical signals, complicating the precise quantification of the target analyte. Common interferences in electrochemical sensing include other vitamins, ions, and organic compounds.
5). What advantages can nanoparticles offer in conductive polymer electrolytes (CPEs)?
Nanomaterials offer an expanded surface area for electrochemical reactions, enhancing the sensor’s sensitivity and efficiency. They can additionally incorporate particular binding sites for the target analyte, hence enhancing the sensor’s selectivity.
For more chemistry blogs, visit chemistry Master