Table of Contents
Preface of CH₃OH:
Artificial photosynthesis is one of the most promising processes in sustainable chemistry. This approach emulates nature’s capacity to transform sunlight into chemical energy, possessing the potential to resolve our energy requirements and environmental issues. A notable use of this technology is the synthesis of methanol (CH₃OH), a clean fuel, via the oxygen-atom-grafting of carbon dioxide (CO₂) onto methane (CH₄). This method, facilitated by a novel AuPd/GaN catalyst, signifies a pivotal advancement in renewable energy production and carbon recycling.
What is the meaning of this, and why is it important? Let us explore chemistry and comprehend how this technology may revolutionise fuel manufacturing.
Comprehending photosynthesis and its consequences:
Photosynthesis is the process by which plants transform sunlight into energy, utilising water and carbon dioxide to produce oxygen and glucose. Artificial photosynthesis aims to emulate this process to generate valuable molecules such as methanol. Methanol is a multifaceted molecule that functions as both an energy source and a chemical feedstock.
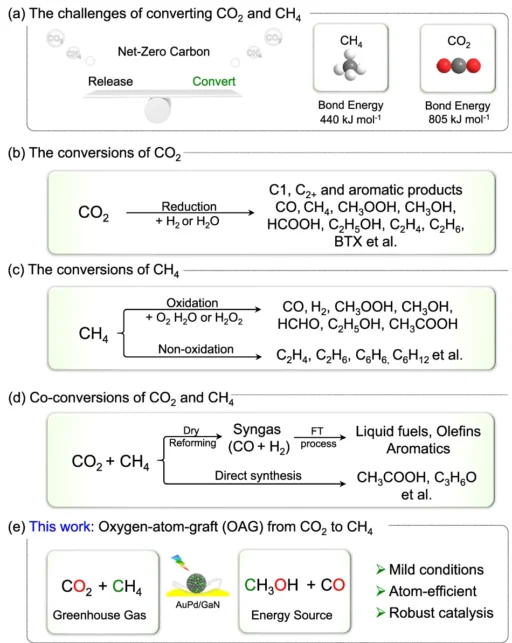
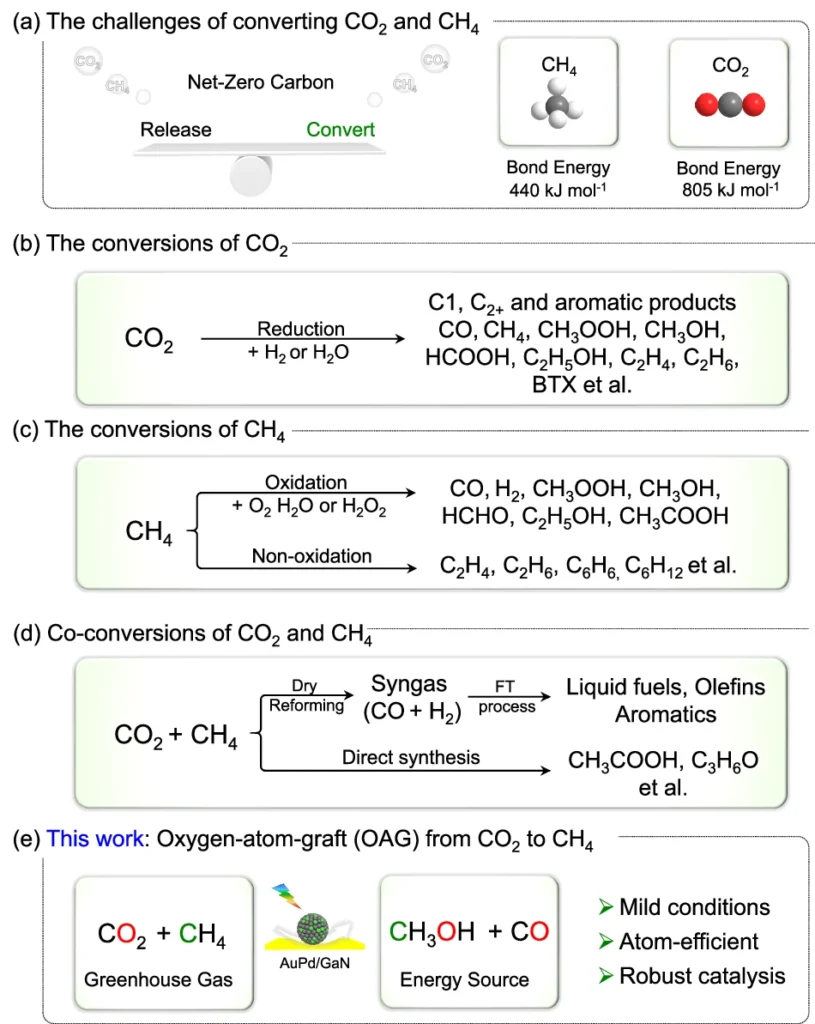
The synthetic variety concentrates on harnessing sunlight to facilitate chemical reactions that diminish CO₂, converting it into more valuable and less detrimental chemicals. In this perspective, synthesising CH2OH from CO2 and CH3 provides an effective method to mitigate greenhouse gas emissions while generating a sustainable fuel supply. Investigating the challenges and approaches for transformations of CO2 and CH4.
Methanol (CH₃OH) and its Industrial Significance:
Industrial applications, such as the production of polymers and pharmaceuticals, extensively use methanol as a potential substitute for gasoline. Its value lies in its ability to function as a clean-burning fuel, emitting fewer toxins than traditional fossil fuels.
The primary appeal of methanol production via CO2 conversion is its embodiment of a circular carbon economy. Rather than emitting detrimental CO₂ into the atmosphere, we may convert it into methanol, completing the cycle and substantially diminishing carbon emissions.
The Difficulty of Transforming CO₂ and CH₄ into CH₃OH:
Although it appears promising, the conversion of CO2 and CH2 into methanol is a formidable challenge. These two molecules exhibit significant stability, indicating they do not readily engage in reactions to generate new compounds. Conventionally, steam reforming, an energy-intensive and inefficient method, produces methanol.
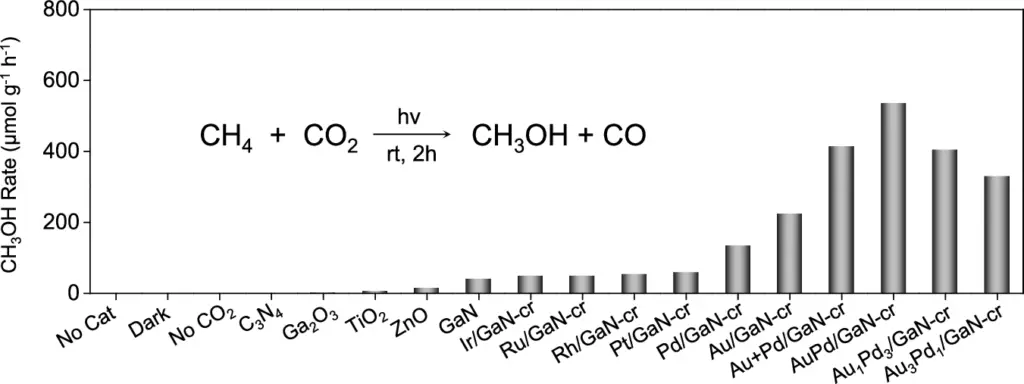
The primary challenge in this innovative method is to selectively transfer an oxygen atom from CO2 to CH2, resulting in methanol while avoiding the formation of undesirable by-products. We use sophisticated catalysts like AuPd/GaN in this process. Methanol production through co-conversion of CH4 and CO2 using an array of semiconductors and metal/GaN-cr interfacial catalysts.
The catalysts play a crucial role in the conversion process from CH to CHOH:
Catalysts are substances that accelerate chemical reactions without being consumed during the process. In this instance, they facilitate the surmounting of the energy barriers linked to the intrinsic stability of CO₂ and CH₄, so simplifying their conversion into methanol.
Gold (Au) and palladium (Pd) are renowned for their catalytic characteristics, especially in oxidation reactions. When combined with gallium nitride (GaN), a semiconductor that absorbs visible light, they create a powerful catalytic system capable of facilitating this intricate chemical reaction.
The Function of AuPd/GaN in Methanol (CH₃OH) Synthesis:
The AuPd/GaN system is essential to this innovative photosynthetic method. Gold and palladium, both noble metals, are proficient catalysts for oxidation processes. Their function is to destabilise the chemical bonds in CO2 and CH2, facilitating the reaction between the two molecules to produce methanol.
Simultaneously, GaN functions as a photosensitive substance. Upon exposure to light, it absorbs photons, supplying the requisite energy to activate the AuPd catalyst. The interplay among these components enhances the overall efficiency of the process. Construction and characterization of AuPd/GaN-ci.
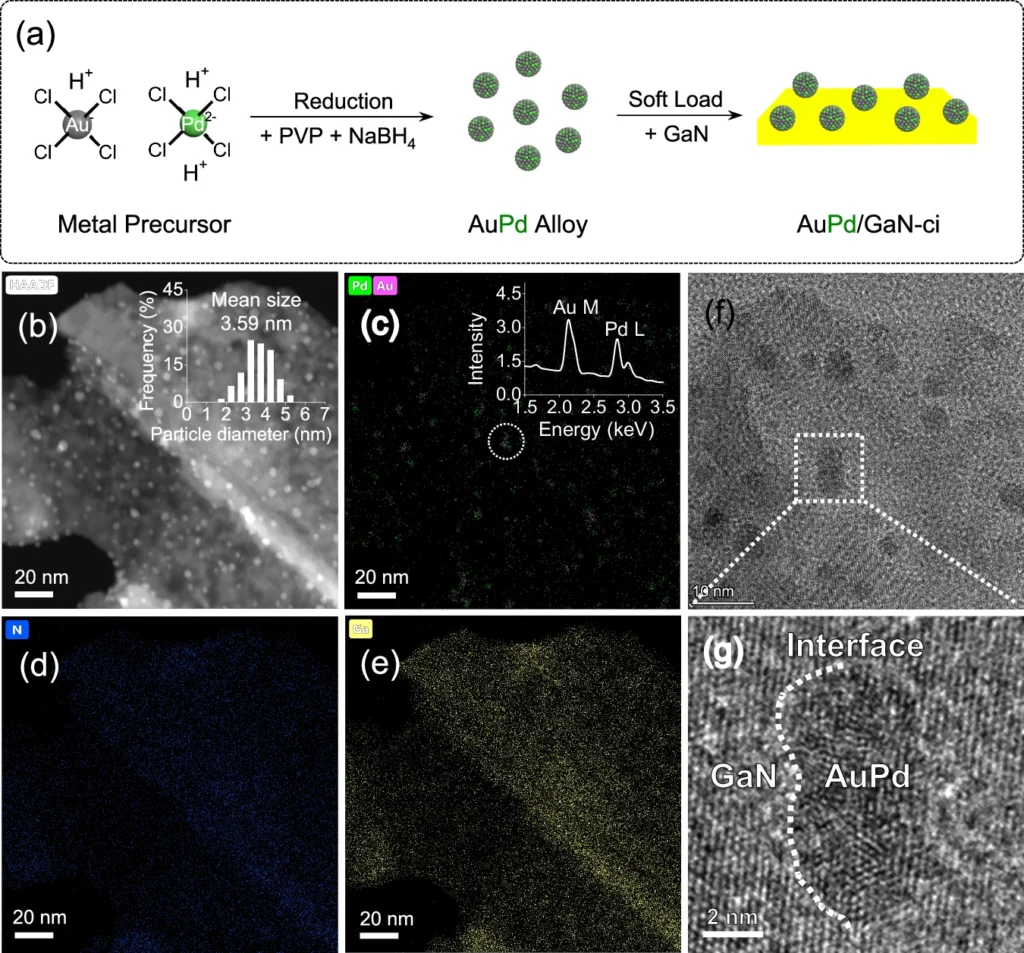
Mechanism of Oxygen Atom Grafting in Carbon Dioxide to Methane Transformation:
The phrase “oxygen-atom-grafting” refers to the transfer of an oxygen atom from CO₂ to CH₄ in this process. The gold-palladium catalyst disrupts the oxygen bonds in CO2, allowing one of its oxygen atoms to attach to a methane molecule. This reaction results in the production of methanol (CHOH) and generates carbon monoxide (CO), which can undergo additional processing.
The AuPd/GaN Catalyst exhibits effectiveness and discrimination:
The selectivity of the AuPd/GaN catalyst is one of its most intriguing features. Selectivity in chemical processes is a catalyst’s capacity to yield a particular product while reducing undesired side reactions. AuPd/GaN exhibits good selectivity for methanol (CH₃OH), effectively converting CO2 and CH2 while minimising the production of by-products such as CO2 or CO. Catalysts and conditions optimization of photoinduced conversion of CH4 with CO2.
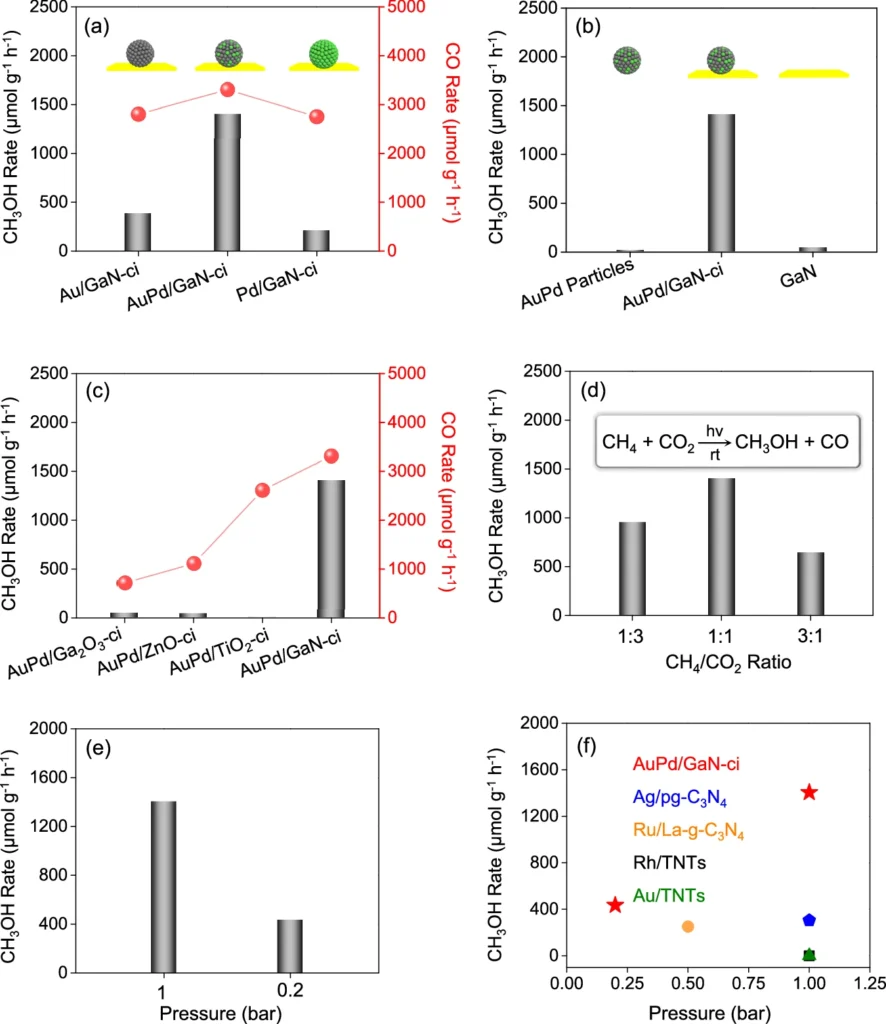
Ecological and financial consequences:
This approach provides simultaneous advantages for both the environment and the economy. The method converts methane, a strong greenhouse gas, into methanol (CH₃OH), thereby mitigating emissions of both CH2 and CO2. The use of methane, which is abundant and frequently flared during natural gas operations, provides an economical feedstock for methanol synthesis.
Benefits of the AuPd/GaN System:
This photosynthetic method of methanol generation has various advantages over conventional techniques.
1). It operates under moderate conditions, reducing the amount of energy used for the reaction.
2). It utilises visible light, which is plentiful and renewable.
3). The AuPd/GaN system exhibits good selectivity, resulting in reduced byproducts and enhanced efficiency. Mechanistic and kinetic study profiles for photoconversion of CH4 and CO2 over AuPd/GaN-ci.
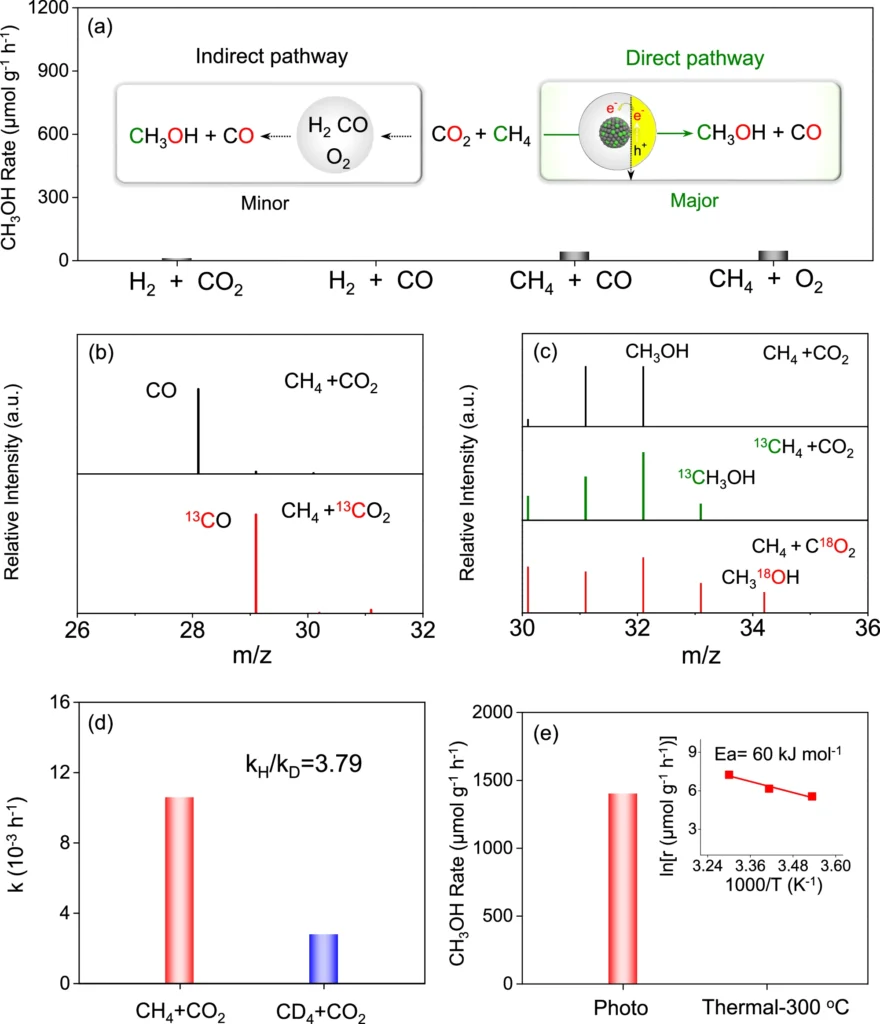
Experimental configuration and parameters:
The AuPd/GaN combination has demonstrated significant potential in experimental studies. Generally, we conduct the reaction at moderate temperatures and atmospheric pressure, parameters that enhance its practicality for industrial applications. Using visible light significantly enhances its sustainability, allowing solar energy to power the process.
Obstacles and Constraints of the Procedure:
Despite its potential, there are still challenges to overcome. Enhancing this method for industrial applications necessitates additional research, especially regarding catalyst durability. Furthermore, managing side effects and maintaining constant catalyst efficacy over time are persistent issues.
Prospective Developments in Artificial Photosynthesis:
The domain of artificial photosynthesis is nascent; however, it possesses significant potential for the future. Advancements in catalyst design and an improved understanding of reaction dynamics could make such technologies more efficient and commercially feasible. Calculation and experiment study profiles of pre-adsorption and activation processes for CH4 and CO2 on the surface of GaN and AuPd/GaN-ci samples.
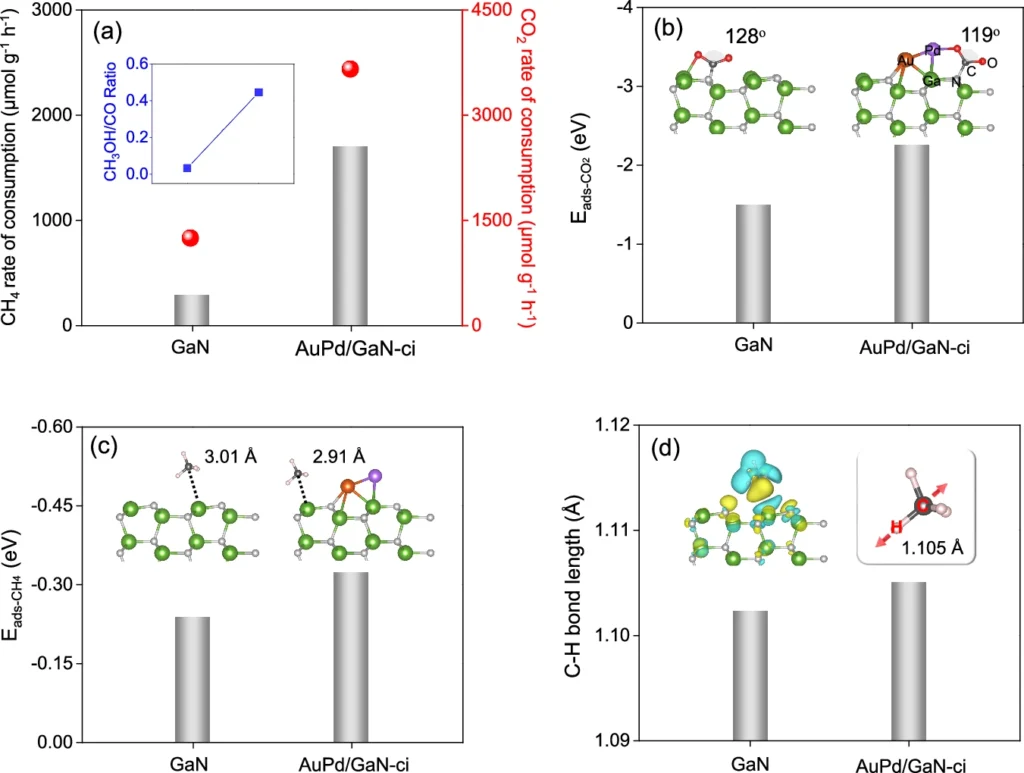
Possible Industrial Applications:
Current chemical manufacturing processes may incorporate this technique, aiding in the reduction of carbon emissions and providing a renewable methanol (CH₃OH) source. It also corresponds with international initiatives to absorb and utilize carbon, positioning it as a pivotal contributor to the advancement of the carbon-neutral industry.
Final Assessment:
The photosynthesis of CH₃OH from CO₂ and CH₄ utilising an AuPd/GaN catalyst signifies significant progress in sustainable chemistry. This approach utilises visible light and a robust catalytic system to address two significant environmental issues: the reduction of greenhouse gases and the production of renewable fuels. This strategy may significantly influence the future of energy production as research progresses.
Frequently Asked Questions:
1). What renders AuPd/GaN an appropriate catalyst for CH₃OH synthesis?
The AuPd/GaN material is very selective, works well, and can work in mild conditions, which makes it perfect for turning CO2 and CH2 into methanol.
2). What is the mechanism for oxygen atom grafting?
During oxygen-atom-grafting, an oxygen atom is moved from CO₂ to CH₄ with the help of an AuPd catalyst. This creates methanol.
3). Can this approach mitigate global CO₂ emissions?
This procedure mitigates greenhouse gas emissions and facilitates carbon recycling by turning CO₂ into useful methanol.
4). What are the current obstacles to this technology’s expansion?
Challenges include catalyst longevity, control of side reactions, and maintaining uniform performance in large applications.
5). How does this methodology contrast with alternative techniques for methanol synthesis?
It is more energy-efficient, environmentally sustainable, and capable of utilising solar energy, rendering it superior to conventional systems dependent on fossil fuels.
For more chemistry blogs, visit chemistry Master