Table of Contents
Preface:
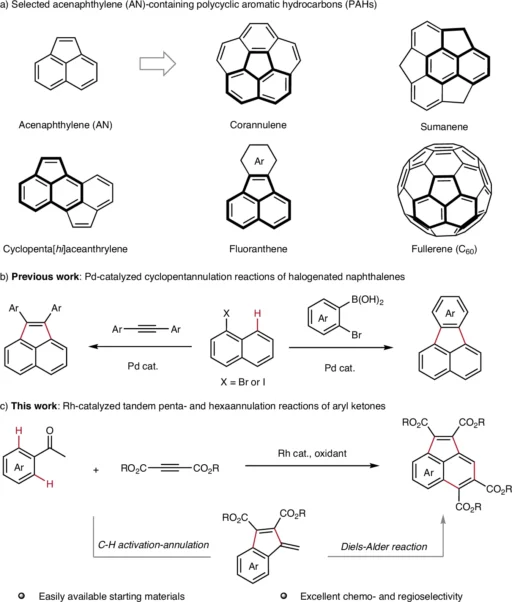
Acenaphthylenes have attracted significant interest in synthetic organic chemistry because of their distinctive structure and diverse uses, ranging from organic electronics to materials research. A notable improvement in their construction is the implementation of C-H activation-based tandem penta- and hexaannulation processes. This technology is more efficient and atom-economic than conventional approaches, significantly decreasing the need for pre-functionalized substrates. This article talks about how to make acenaphthene by activating the C-H bond, focusing on the transformative tandem penta- and hexaannulation reactions.
What are acenaphthylenes?
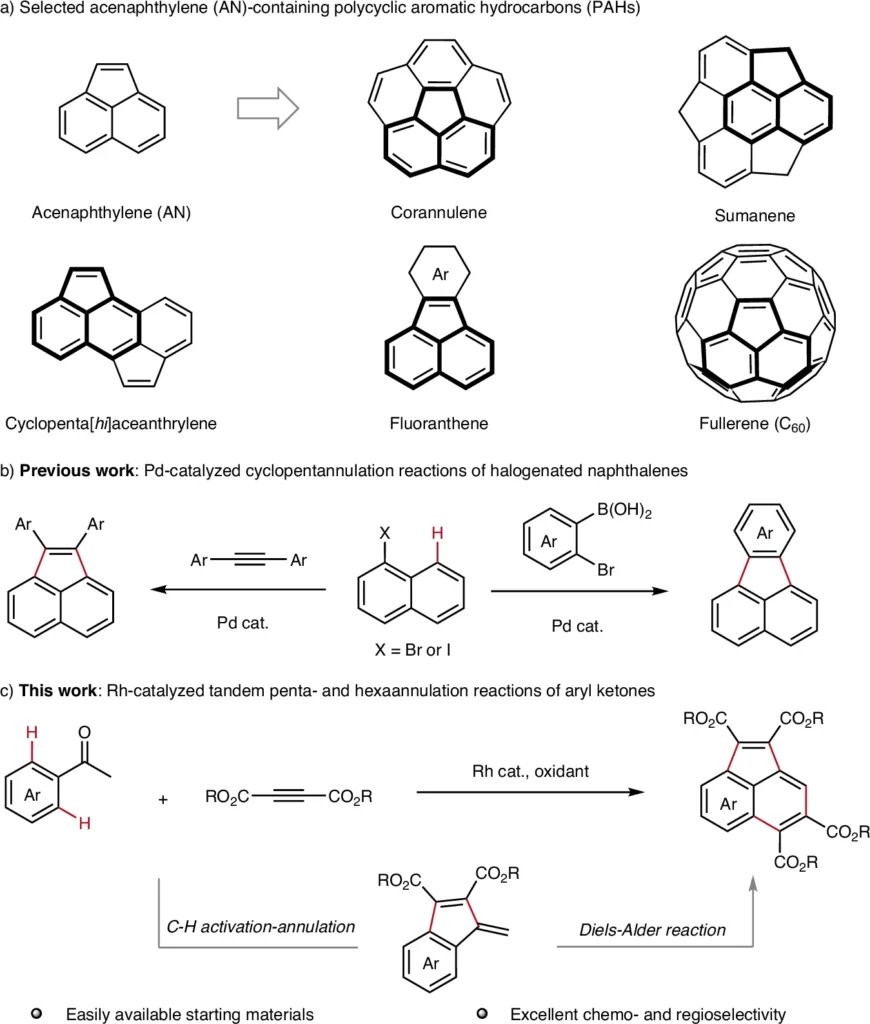
Acenaphthylenes are polycyclic aromatic hydrocarbons (PAHs) with a fused ring structure. They are made up of benzene and cyclopentene. Their inflexible, planar configuration imparts distinctive characteristics, rendering them indispensable in domains such as organic semiconductors, photodetectors, and light-emitting diodes (LEDs). They serve as essential intermediates in the synthesis of bigger polycyclic aromatic hydrocarbons (PAHs) and are present in natural substances such as coal tar.
Researchers thoroughly investigate acenaphthylenes for applications in organic electronics because their conjugated arrangements facilitate intriguing electrical properties. Nonetheless, these compounds’ efficient and sustainable synthesis has historically posed challenges—until the emergence of C−H activation-based techniques. Acenaphthylene (AN) contains polycyclic aromatic hydrocarbons (PAHs) and their synthetic strategies.
The importance of C-H activation in organic synthesis:
In conventional organic synthesis, the functionalization of a molecule often necessitates several steps utilizing pre-functionalized starting materials. Chemists can directly interact with carbon-hydrogen bonds through C−H activation, turning them into reactive sites without needing pre-functionalization. This approach has emerged as a fundamental element in contemporary synthetic strategies owing to its ability to optimize reactions, reduce waste, and enhance efficiency.
The elegance of C-H activation lies in its ability to directly exploit C-H bonds, which are abundant yet frequently unreactive in organic compounds. Catalysts, which are typically transition metals, can activate these bonds and facilitate a variety of transformations, including the synthesis of acetylenes. Optimization of the reaction conditions.
An Overview of Tandem Annulation Reactions:
Tandem annulation reactions synthesize two or more rings in a single sequence of stages, typically involving cyclization and the creation of multiple bonds. Tandem penta- and hexaannulation reactions in acenaphthylenes create five- and six-membered rings by activating C-H groups at the same time or one after the other.
Tandem annulations are a one-pot synthesis method that significantly improves efficiency compared to conventional annulation techniques, which typically necessitate numerous reactions and isolations. These reactions are particularly advantageous for the synthesis of intricate polycyclic systems such as acenaphthylenes.
Mechanistic Understanding of C-H Activation-Driven Annulation:
Transition-metal catalysis, which uses metals like palladium, rhodium, or ruthenium, primarily facilitates C-H activation. These metals interact with the substrate, enabling selective cleavage of the C−H bond and incorporation into the molecule, thereby promoting further cyclization and ring formation.
The procedure for Penta- and hexaannulation reactions typically entails:
1). The coordination of a metal to a guiding group or substrate results in the breakage of C-H bonds.
2). Insertion into a π-system (e.g., alkyne or alkene)
3). Cyclization is necessary to obtain the desired polycyclic product.
4). This technique offers significant selectivity and efficiency, frequently yielding the required acenaphthylenes in exceptional quantities. Bidirectional annulation reaction.
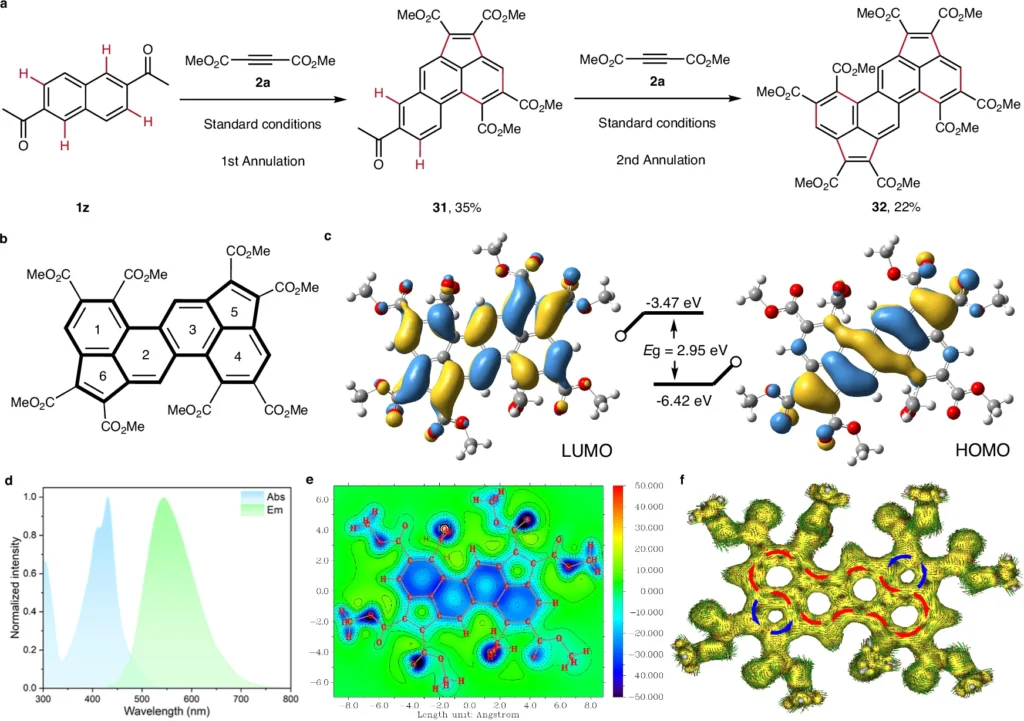
Pentaannulation reactions in Acenaphthylene Synthesis:
Pentaannulation denotes the synthesis of five-membered rings, an essential process in the assembly of acenaphthylenes. We commonly use palladium or rhodium catalysts to promote the synthesis of a cyclopentadiene moiety, which integrates into the acenaphthylene structure.
A common way is for an aromatic substrate to react with an alkyne, which is then followed by C–H activation and cyclization to make a ring with five members. This technique is exceptionally effective, frequently yielding near-quantitative results with significant functional group compatibility.
Hexannulation reactions in Acenaphthylene Synthesis:
Hexaannulation is the process of synthesizing six-membered rings. This phase frequently enhances the pentaannulation process, contributing further complexity and structural diversity to the final acenaphthylene product.
Ruthenium and rhodium catalysts are often used for hexaannulation, which makes it easier to make a benzene ring that is joined to an acenaphthylene framework. This tandem reaction has remarkable efficiency and is especially advantageous for the synthesis of acenaphthylenes featuring extensive π-conjugation. Mechanism study.
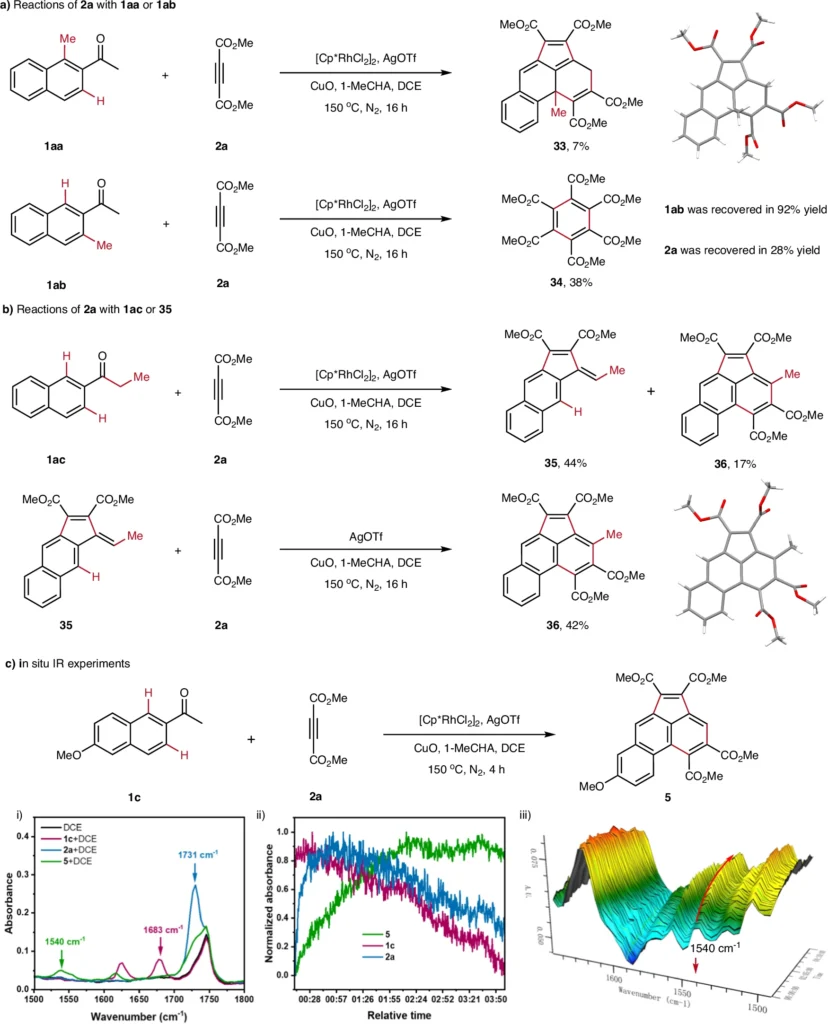
Obstacles in C−H Activation-Driven Tandem Annulation Reactions:
Despite its advantages, C-H activation-based tandem annulation poses certain challenges. The biggest problems are with stereoselectivity (making sure that activation happens at the right C-H bond) and regioselectivity (making sure that activation happens at the right C-H bond).
Another issue concerns catalyst efficiency. Palladium and rhodium are highly effective but can be costly and may occasionally induce side reactions, hence diminishing the overall yield.
Approaches to Conquer Synthetic Obstacles:
In response to these challenges, chemists have devised sophisticated ligands to improve the selectivity of C-H activation processes. Furthermore, chemists consistently devise novel catalytic systems to enhance efficiency, decrease expenses, and expand the substrate range.
For example, using bimetallic catalysts has shown promise in increasing regioselectivity, and changing ligands have helped make these processes more functional and group-tolerant.
Industrial Applications of Acetylenes:
Acenaphthylenes are in high demand for the creation of organic semiconductors, luminescent compounds, and sophisticated polymers. Their capacity to carry energy and emit light renders them optimal for application in organic LEDs and photovoltaic systems.
In materials science, they serve as foundational components for the synthesis of larger polycyclic aromatic hydrocarbons (PAHs), which are critical for the advancement of molecular electronics and nanoscale devices.
The environmental and economic consequences of C+H activation:
C-H activation provides a more sustainable alternative to conventional synthetic processes by frequently decreasing the number of required stages and minimizing waste. Although nascent, the use of abundant earth metals is gaining momentum, potentially reducing the environmental and economic burdens associated with pricier transition metals like palladium.
Case Studies: Effective Synthesis of Acenaphthylenes
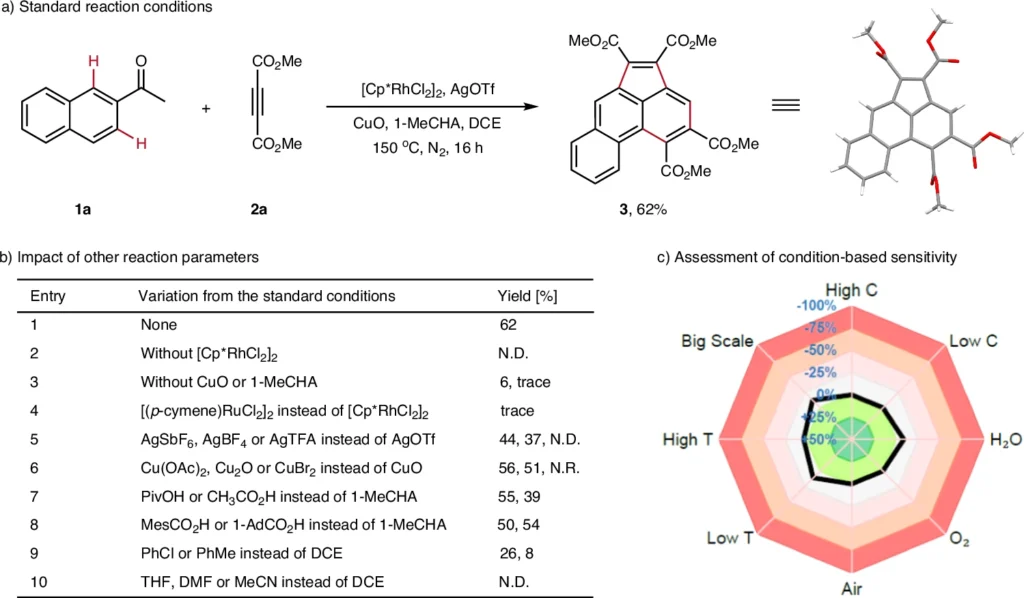
Recent scholarly research has proven the efficacy of tandem penta- and hexaannulation reactions. A 2021 study demonstrated the efficacy of a rhodium-catalyzed tandem annulation, which yielded acenaphthylenes in near-quantitative amounts. Proposed mechanism and stepwise annulation reactions.
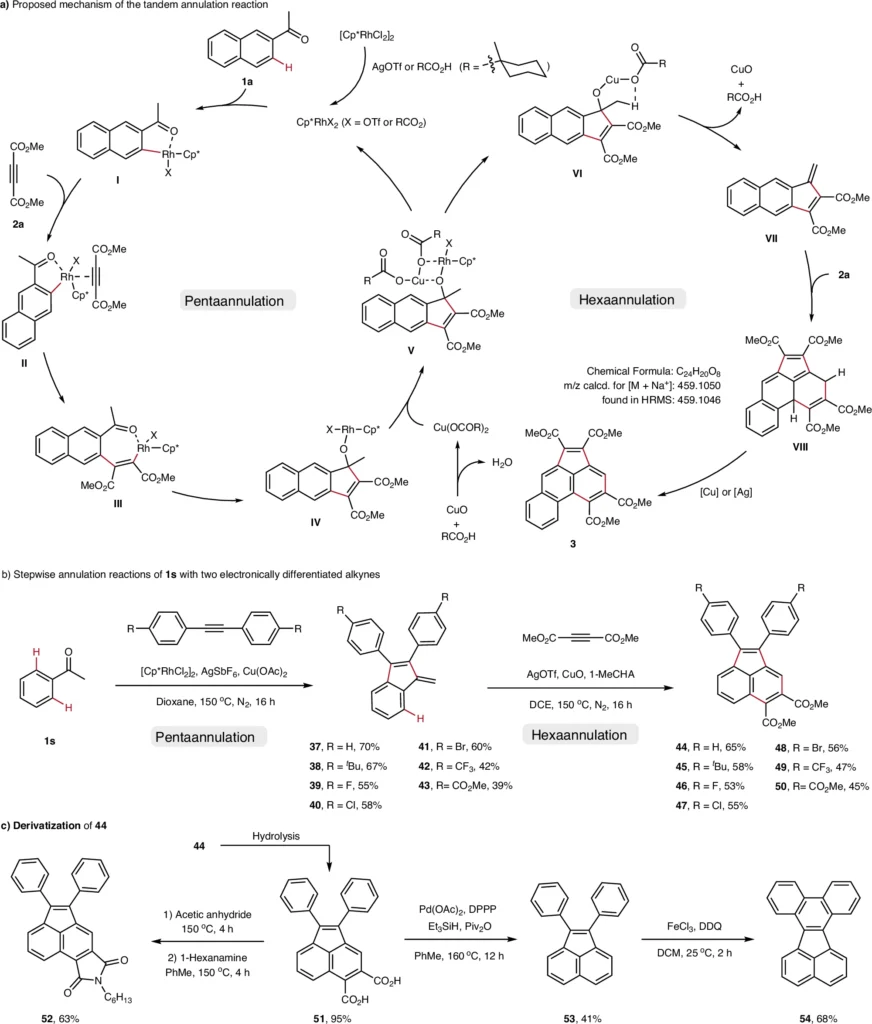
Prospective Avenues in Acenaphthylene Synthesis:
The advancement of acenaphthylene synthesis depends on the ongoing evolution of novel C-H activation methodologies. The potential for more intricate polycyclic structures and applications in advanced technology primes the topic for future investigation.
Comparison of Penta- and Hexaannulation Techniques:
Pentaannulation is typically more straightforward, whereas hexaannulation introduces greater structural complexity. The procedure selection is contingent on the intended characteristics of the final acenaphthylene, as each has its advantages.
Final Assessment:
The creation of acenaphthylenes through tandem penta- and hexaannulation reactions driven by C–H activation is a big step forward in organic synthesis. Employing these techniques, chemists can effectively synthesize intricate polycyclic materials with elevated yields and reduced waste. As research progresses, we may anticipate the emergence of increasingly novel methodologies, thus expanding the limits of synthetic chemistry.
Frequently Asked Questions:
1). What are the primary applications of acetylenes?
Organic electronics, which include semiconductors, LEDs, and photovoltaic devices, predominantly use acenaphthylenes.
2). What are the advantages of C−H activation compared to conventional methods?
C−H activation exhibits enhanced efficiency, diminishing the necessity for prefunctionalized substrates and minimizing waste.
3). What distinguishes pentaannulation from hexaannulation?
Pentaannulation produces five-membered rings, whereas hexaannulation yields six-membered rings, thereby increasing structural complexity.
4). What are the commonly used catalysts in these reactions?
Palladium, rhodium, and ruthenium are the predominant catalysts employed in C-H activation-based annulation processes.
5). Does C−H activation confer environmental advantages?
Indeed, C−H activation is more sustainable and atom-efficient, minimizing waste and the number of necessary synthetic steps.
For more chemistry blogs, visit chemistry Master