Table of Contents
Overview:
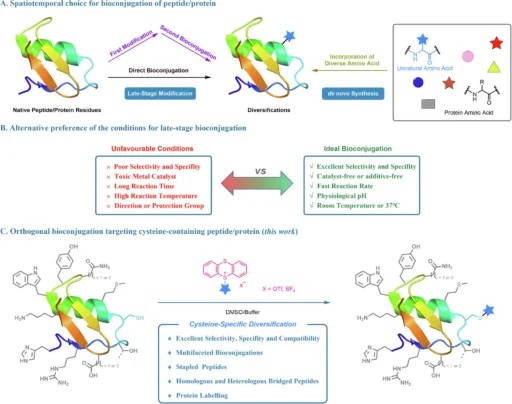
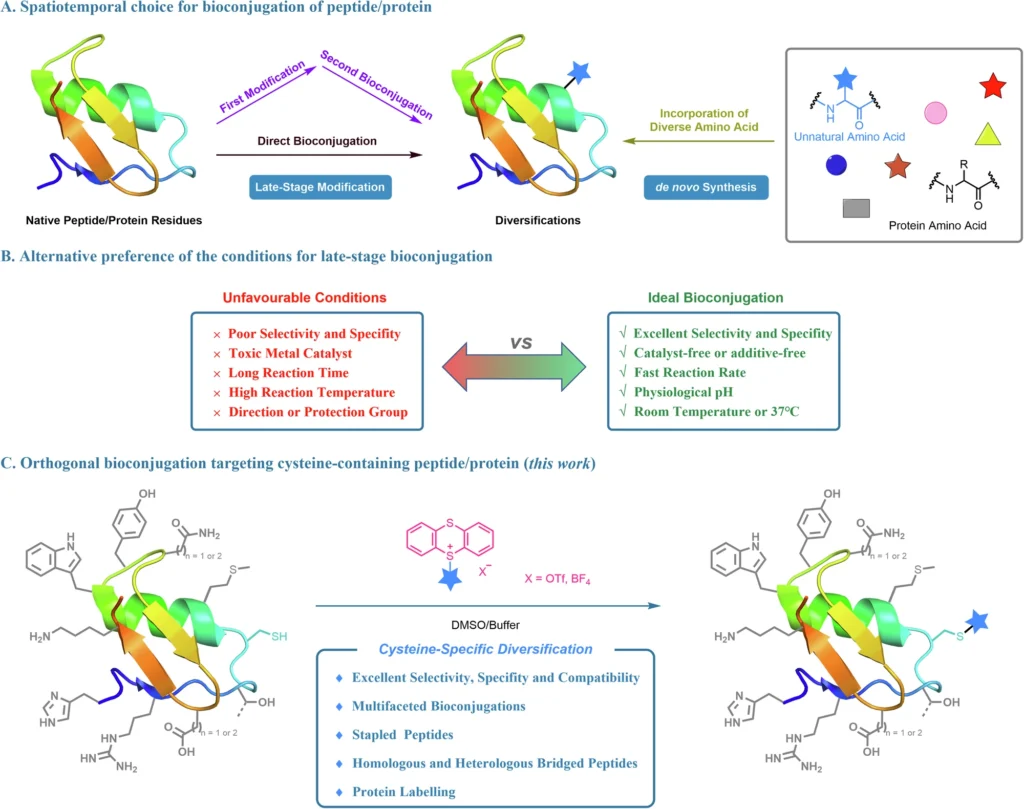
Bioconjugation, a highly effective technique in the dynamic field of biochemistry, is used to change peptides and proteins. It plays a crucial role in advancing drug development, diagnostics, and therapeutic interventions. Cysteine is particularly notable among the several targets for bioconjugation because of its distinctive reactivity. Alkyl thianthrenium salts have recently become a revolutionary reagent in cysteine-targeted bioconjugation. They provide orthogonality and specificity that were previously difficult to attain.
Comprehending the bioconjugation process:
Bioconjugation is a chemical technique that involves the covalent bonding of two molecules, usually one of which is a biomolecule such as a peptide or protein. This approach is essential in various applications, such as the advancement of antibody-drug conjugates, protein labeling, and the production of therapeutic peptides. Researchers can manipulate the characteristics, stability, and interactions of proteins or peptides by attaching functional groups or medicines to specific locations. This can lead to the creation of new therapies and diagnostic tools. Ideal chemical bioconjugation.
The Significance of Cysteine in Bioconjugation:
Cysteine is an amino acid that possesses a thiol group (-SH), known for its strong reactivity and ability to establish stable connections with many chemical entities. The reactivity of cysteine makes it a very suitable target for bioconjugation. Cysteine residues are frequently located strategically in proteins and peptides, allowing for targeted changes. The thiol group is capable of undergoing nucleophilic attack, resulting in the creation of covalent bonds with electrophilic chemicals. This process serves as a fundamental principle in numerous bioconjugation techniques.
Difficulties in Cysteine-Targeted Bioconjugation:
Although cysteine-targeted bioconjugation has benefits, the process is not without difficulties. Selectivity is an important aspect to consider. Cystine’s strong reactivity can occasionally result in unintentional changes to other nucleophilic sites within the protein or peptide. In addition, the possibility of side reactions adds complexity to the process, necessitating the use of technologies that are both highly specific and independent from other functional groups in the biomolecule.
An Overview of Alkyl Thianthrenium Salts:
Alkyl thianthrenium salts are currently receiving significant interest as a powerful tool in the field of bioconjugation, specifically for targeting cysteine residues. These salts have a sulfur-containing thianthrene ring that promotes alkylation reactions. Alkyl thianthrenium salts, due to their unique composition, can specifically modify cysteine residues through alkylation while leaving other amino acids unaffected. This property makes them ideal for orthogonal bioconjugation. Orthogonal bioconjugation targeting bioactive peptides using thianthrenium saltsa.
Mode of operation:
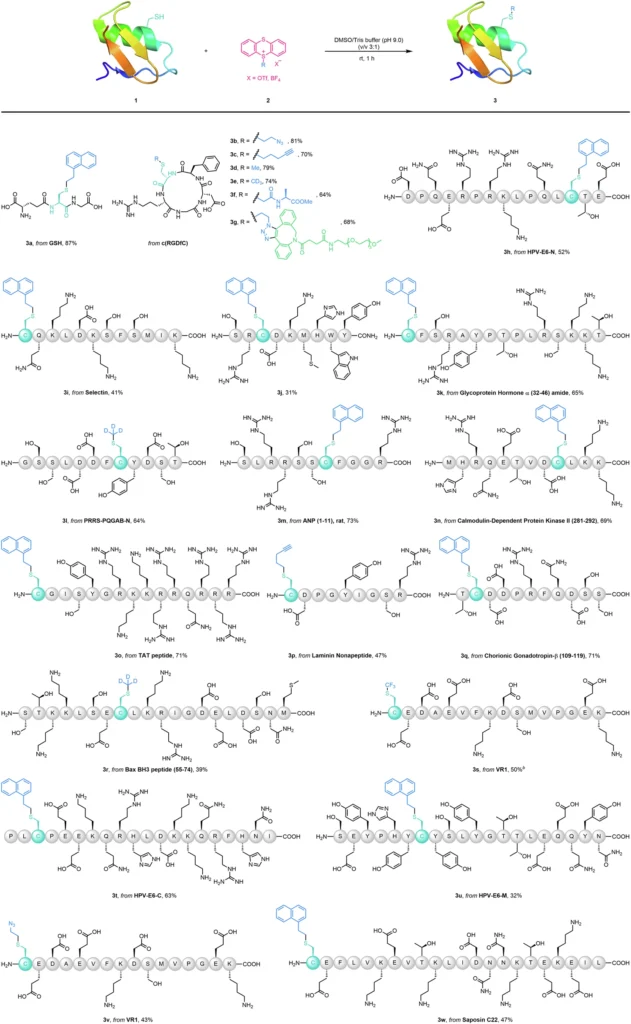
The alkyl thianthrenium salts efficiently and elegantly modify cysteine by a specific method. The thianthrenium ring structure stabilizes the reactive alkyl group, restricting its reactivity to only the cysteine’s thiol group. Upon encountering a cysteine residue, the alkyl group nucleophilically substitutes with the thiol group, forming a robust covalent bond. The reaction has a high degree of specificity towards cysteine, mostly because of the distinctive reactivity of the thiol group under physiological circumstances.
Orthogonality in Bioconjugation:
The concept of orthogonality in bioconjugation refers to the ability to selectively and independently modify different biomolecules without interfering with each other.
Orthogonality in bioconjugation refers to a chemical reaction’s ability to occur in the presence of other functional groups without causing any interference. In this case, alkyl thianthrenium salts show this idea by focusing on cysteine residues and staying away from other amino acids or functional groups in the biomolecule. The significant level of orthogonality is especially advantageous in intricate biological systems that may contain many reactive sites.
Benefits of Utilizing Alkyl Thianthrenium Salts:
When used in cysteine-targeted bioconjugation. One of their main advantages is their great selectivity, which reduces the likelihood of unintended alterations. Precision is of utmost importance when dealing with intricate proteins or peptides. The strong thianthrenium-cysteine bond also ensures that the change lasts for a long time, which is important for uses that need long-term stability, like imaging inside living things or drug delivery. Orthogonal construction of stapled and bridged peptides
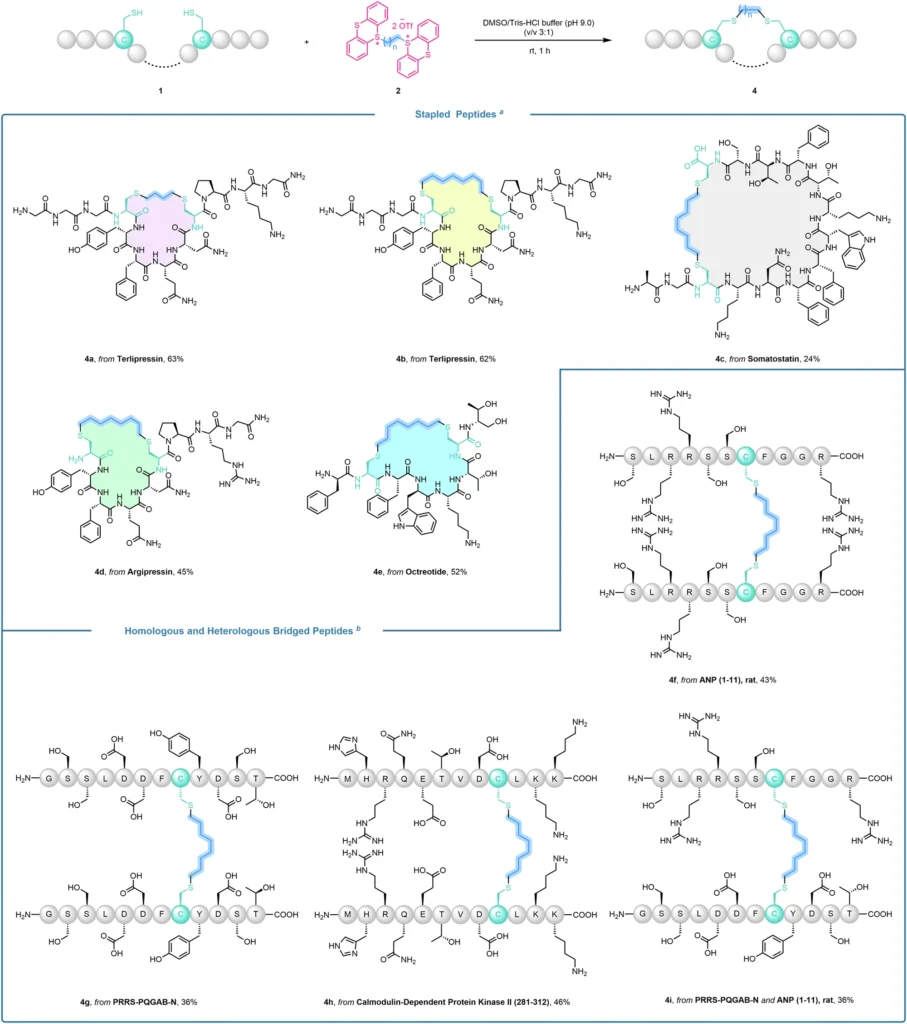
Peptide modification applications:
Alkyl thianthrenium salts have significant potential for modifying therapeutic peptides. Researchers can improve the stability, bioavailability, and effectiveness of peptides used in therapy for different diseases by specifically altering cysteine residues. Researchers can shield a peptide medication from protease degradation by altering its cysteine residues. This alteration leads to an increase in the drug’s half-life in the bloodstream and enhances its therapeutic efficacy.
Protein modification applications:
Researchers are currently exploring the potential of alkyl thianthrenium salts for protein modification. Researchers are specifically studying their potential in developing precise drug delivery systems and marking proteins for imaging and tracking in biological systems. By chemically bonding a medicinal molecule to a particular cysteine residue on a protein, scientists can precisely guide the medicine to certain tissues or cells, therefore minimizing unintended consequences and improving the treatment’s effectiveness. This methodology is especially advantageous in the advancement of precision medicine because the objective is to customize therapies for specific patients according to their distinct biological composition. Multifaceted bioconjugation through the click reactiona.
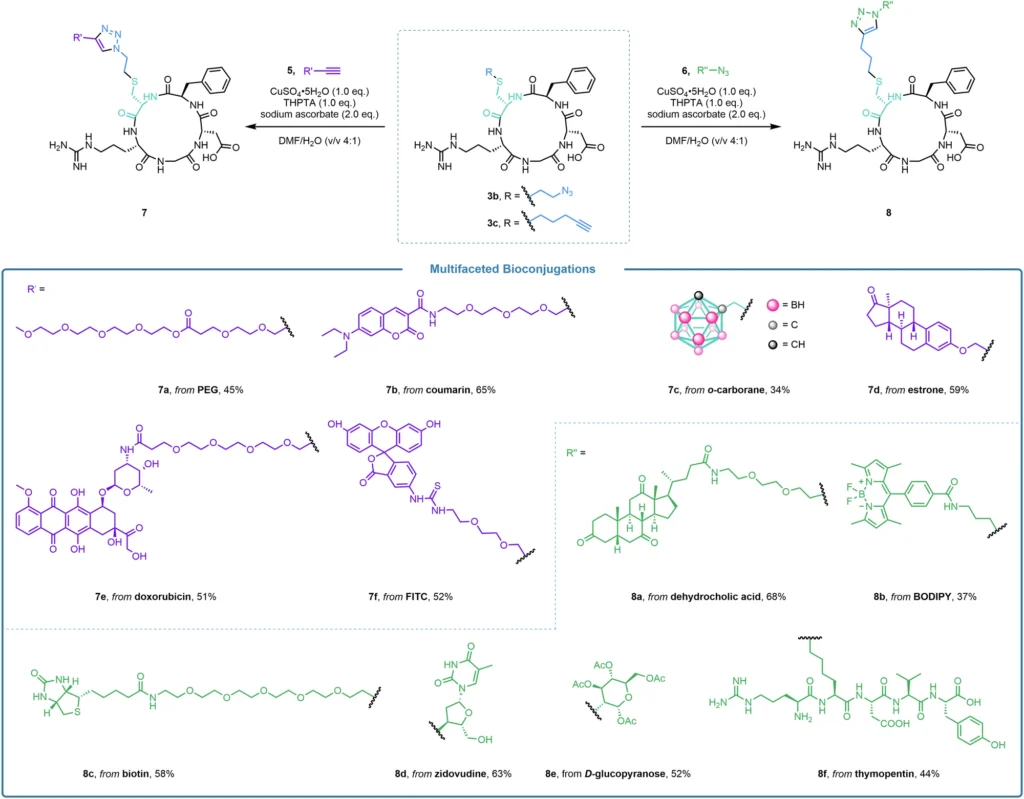
Comparative Analysis of Bioconjugation Techniques:
There are some special benefits that alkyl thianthrenium salts have over other bioconjugation methods, like those that use iodoacetamides or maleimides. We employ iodoacetamides for cysteine modification; however, they may display reduced selectivity and react with other nucleophiles in the protein. Maleimides are commonly used for bioconjugation with thiols, but they can break down in water, which makes them unstable. Conversely, alkyl thianthrenium salts offer a more stable and selective option, making them more desirable in various situations.
The experiment must take factors into account:
When employing alkyl thianthrenium salts for bioconjugation, it is crucial to meticulously choose the reaction conditions to optimize the yield and specificity. Various factors, including pH, temperature, and solvent, can influence the effectiveness of the reaction. It is also important to take into account the concentration of both the protein or peptide and the alkyl thianthrenium salt, as well as the possibility of competing reactions in the presence of other nucleophiles. By optimizing these factors, researchers can achieve higher yields while simultaneously reducing unwanted side effects. Multifaceted modifications of BSAa.
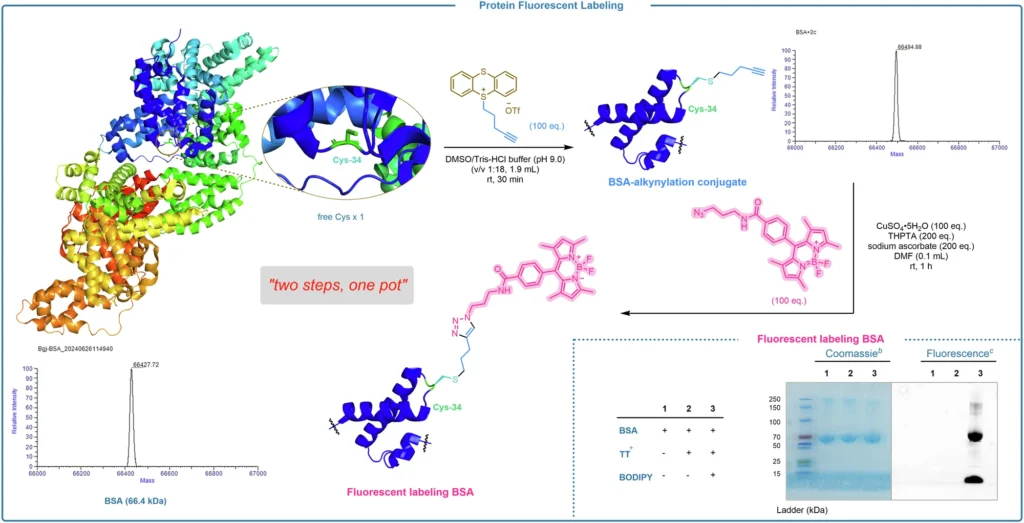
Constraints and obstacles:
Although alkyl thianthrenium salts offer numerous benefits, their application in bioconjugation is not without constraints. An important concern is the capacity to scale up the reaction for large-scale production, which may necessitate additional optimization. Also, even though the selectivity for cysteine is high, it can still be hard to work with complex systems that have a lot of cysteine residues, so the reaction conditions need to be carefully managed. Current research aims to tackle these issues and broaden the application of alkyl thianthrenium salts in other biological scenarios.
Potential Future Developments in Orthogonal Bioconjugation:
The prospects for orthogonal bioconjugation, specifically with alkyl thianthrenium salts, appear to be encouraging. Current trends include the creation of novel alkylating chemicals capable of selectively targeting alternative amino acids and the use of these techniques in more intricate biological systems. These techniques have immense potential for practical applications, particularly in targeted and precision medicine fields. They can provide more efficient and minimally intrusive treatments.
In conclusion:
It is a big step forward in the field of biochemical modification that alkyl thianthrenium salts can be used for orthogonal bioconjugation, specifically on peptides and proteins that contain cysteine. Their exceptional selectivity, stability, and orthogonality make them influential instruments for researchers aiming to precisely and reliably change biomolecules. As ongoing research progresses, we anticipate the emergence of additional inventive applications and enhanced techniques that will further augment the possibilities of bioconjugation in the field of biomedical science.
Frequently Asked Questions:
1). Why is cysteine a common target for bioconjugation?
The unique thiol group of cysteine, which is incredibly reactive and allows for specific alteration in proteins and peptides, makes it preferred.
2). What is the comparative efficacy of alkyl thianthrenium salts compared to other alkylating agents?
When it comes to bioconjugation targeting cysteine, alkyl thianthrenium salts are better than iodoacetamides and maleimides because they are more stable and selective.
3). What are the possible adverse effects of utilizing alkyl thianthrenium salts in living organisms?
Despite being generally considered safe, there is a possibility of off-target changes if not adequately regulated, although this is less likely due to their high specificity.
4). Are alkyl thianthrenium salts suitable for altering other amino acids?
At present, they demonstrate optimal efficacy for cysteine, but continuing research aims to broaden their application to additional amino acids.
5). What are the most favorable applications of this approach in drug development?
Targeted drug delivery systems, increasing the stability of peptides, and making new therapeutic agents with better specificity and effectiveness are all possible uses that look promising.
For more chemistry blogs, visit chemistry Master