Table of Contents
Modern culture widely uses lithium-ion batteries, also referred to as Li-ion batteries, to power a variety of devices such as smartphones, electric vehicles, and renewable energy storage systems. The current collector is a crucial component that enables the movement of electrons during the process of charging and discharging batteries. Aluminium (Al) is a notable choice for current collectors due to its lightweight nature, affordability, and exceptional electrical conductivity. Nevertheless, current collectors face issues due to their vulnerability to passivation and corrosion, which can undermine the performance and durability of batteries.
Overview of the concepts of passivation and corrosion:
Passivation is a process that involves treating a material or surface to make it less reactive and more resistant to corrosion.
a). Passivation is the process by which a metal naturally develops a thin coating of oxide on its surface when it comes into contact with oxygen or moisture. The oxide layer functions as a protective barrier, inhibiting more oxidation of the underlying metal.
b). Corrosion refers to the process of gradual deterioration or destruction of a material, typically a metal, due to chemical reactions with its environment.
Chemical or electrochemical reactions with its surroundings cause a metal to gradually deteriorate. The consequences include material loss and degradation of mechanical qualities, ultimately resulting in metal component failure.
The importance of aluminum current collectors in lithium-ion batteries is significant:
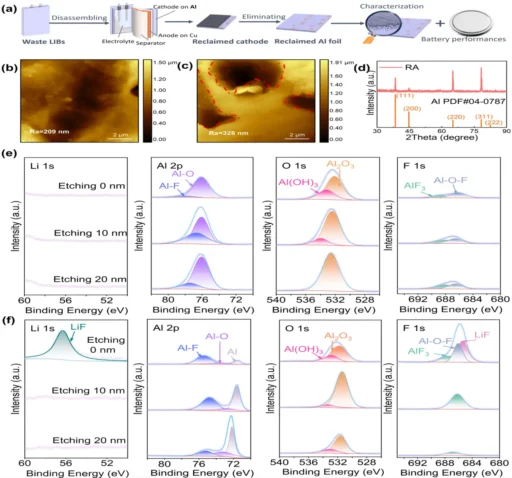
Aluminum current collectors are essential for ensuring the optimal performance and dependability of Li-ion batteries. Aluminum current collectors function as the conductive framework for electrode materials, enabling efficient electron transportation during charge and discharge cycles. Furthermore, the capacity of Aluminum current collectors to resist corrosion is crucial for guaranteeing the durability and safety of Li-ion batteries over an extended period. Schematics and characterization of the samples.
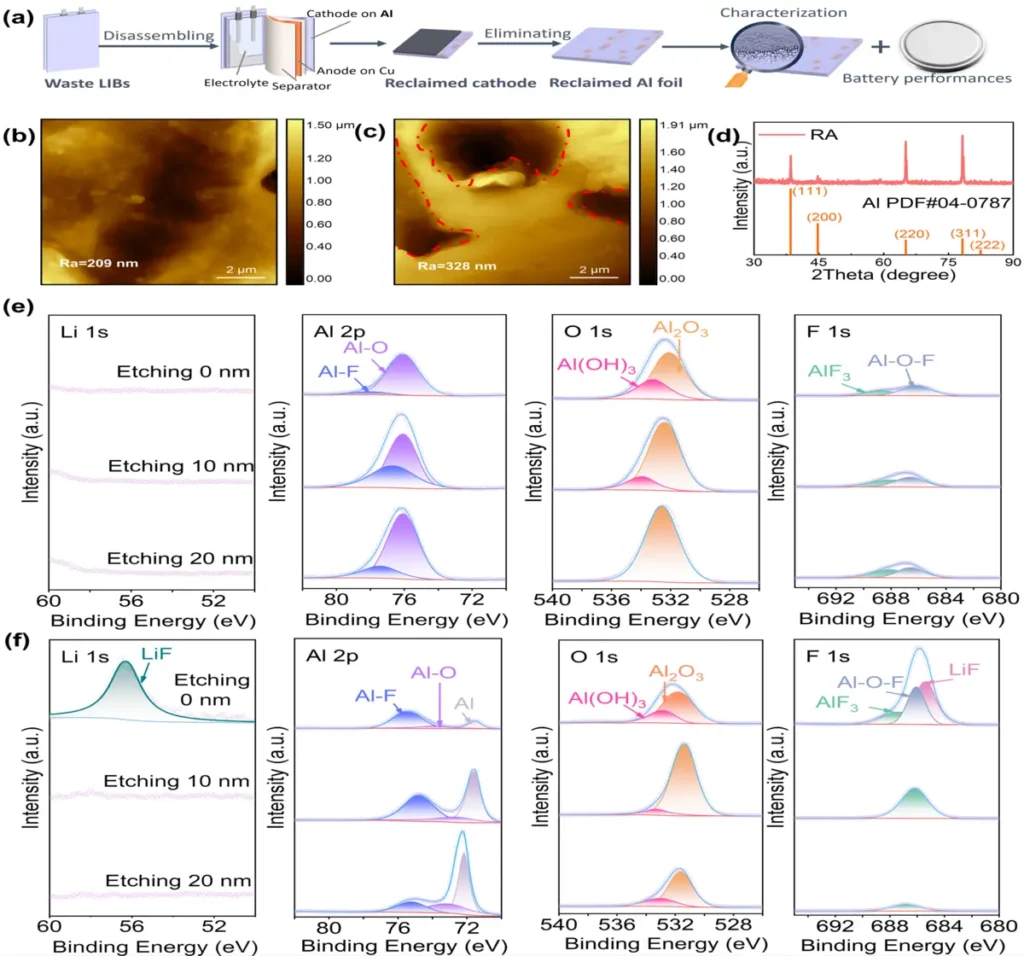
Passivation mechanisms in Aluminum current collectors:
The Aluminum current collectors undergo passivation by developing a thin layer of oxide, predominantly aluminum oxide (Al2O3), on their surface. Aluminum reacts with oxygen or water vapor in the atmosphere to trigger this process, creating stable oxide compounds that protect the underlying metal from further oxidation.
Corrosion mechanisms in Aluminum current collectors:
Although Aluminum current collectors have a protective oxide layer, they can still corrode under certain conditions. Factors such as exposure to aggressive electrolytes, elevated temperatures, and mechanical stress can accelerate corrosion processes. This acceleration can cause the breakdown of the oxide layer and the subsequent degradation of the metal through electrochemical reactions.
Effects of Passivation and Corrosion on Battery Performance:
This study examines the impact of passivation and corrosion on battery performance.
The passivation and corrosion of aluminum current collectors can have significant consequences for the efficiency and durability of lithium-ion batteries.
1). Effect on Conductivity:
Passivation layers can enhance the impedance at the electrode-electrolyte interface, thereby diminishing the total conductivity of the battery and impacting its charge-discharge efficiency.
2). Influence on Battery Lifespan:
Corrosion-induced degradation of aluminum current collectors can negatively affect a battery’s lifespan. This degradation can cause mechanical failure and loss of electrical contact, leading to capacity fade and a decrease in the number of cycles the battery can undergo. Battery performances of LiCoO2 on different Al foils.
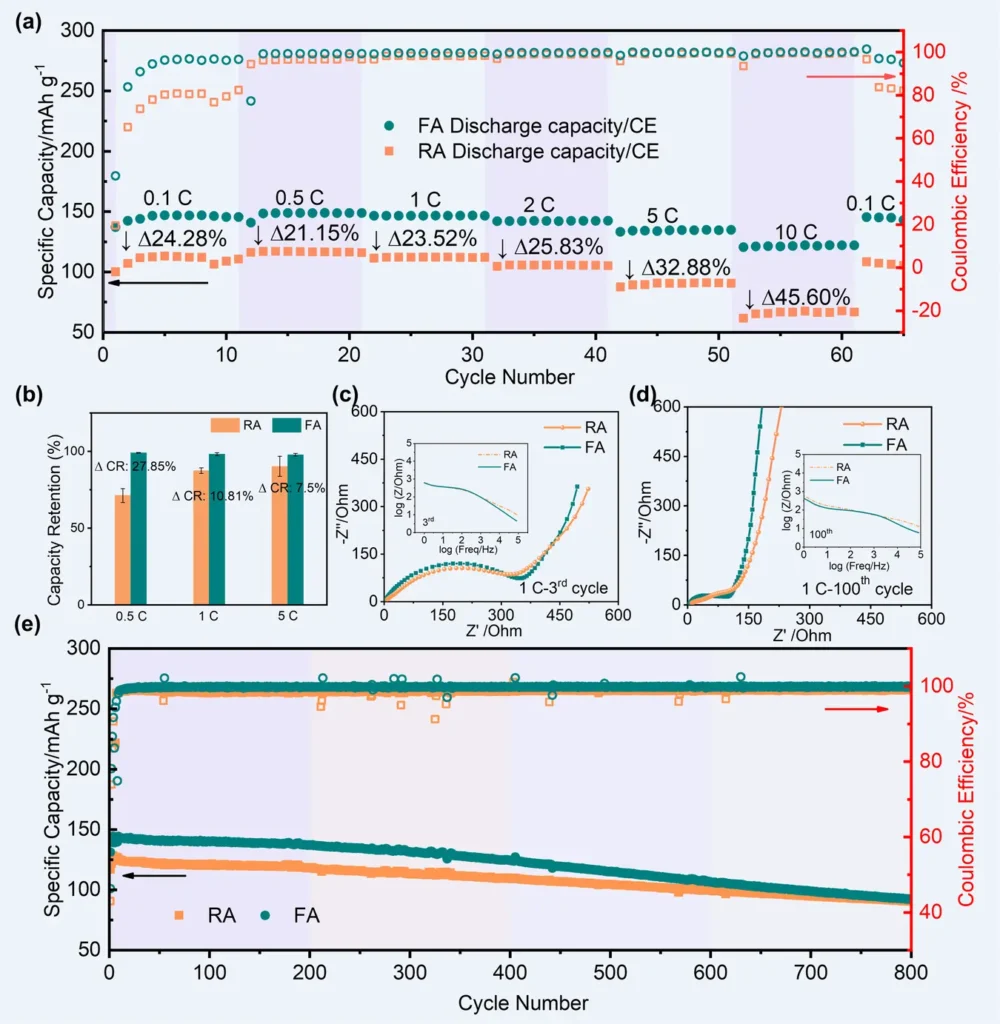
Techniques for Improving Passivation and Resistance to Corrosion:
To address the problems of passivation and corrosion in aluminum current collectors, several surface treatments and alloying procedures have been developed.
1). Surface treatments:
Applying protective layers to the surface of Aluminum current collectors, such as anodization or chemical vapor deposition, can improve their corrosion resistance by creating an extra barrier against environmental influences.
2). Methods of Alloying:
Combining aluminum with other metals like zinc or magnesium modifies the composition and microstructure of the oxide layer, enhancing its stability and adhesion to the underlying metal substrate.
3). Nanostructuring:
Nanostructuring refers to the process of creating materials or structures with nanometer-scale dimensions.
By producing nano-sized characteristics on the surface of Al current collectors through nanostructuring techniques like electrodeposition or physical vapor deposition, we can increase their mechanical durability and corrosion resistance.
Exploration and advancement in the fields of passivation and corrosion reduction:
We are conducting ongoing research and development to enhance the passivation and corrosion resistance of aluminum current collectors.
1). Latest Progress:
Advancements in materials science and surface engineering have recently led to the creation of new coatings and nanostructured materials with exceptional corrosion resistance and mechanical properties.
2). Prospects for the future:
By incorporating sophisticated characterization methods like electron microscopy and spectroscopy, we anticipate gaining a fresh understanding of the processes of passivation and corrosion in aluminum current collectors. This knowledge will help to develop more efficient strategies to prevent these issues . Characterization of the corroded species.
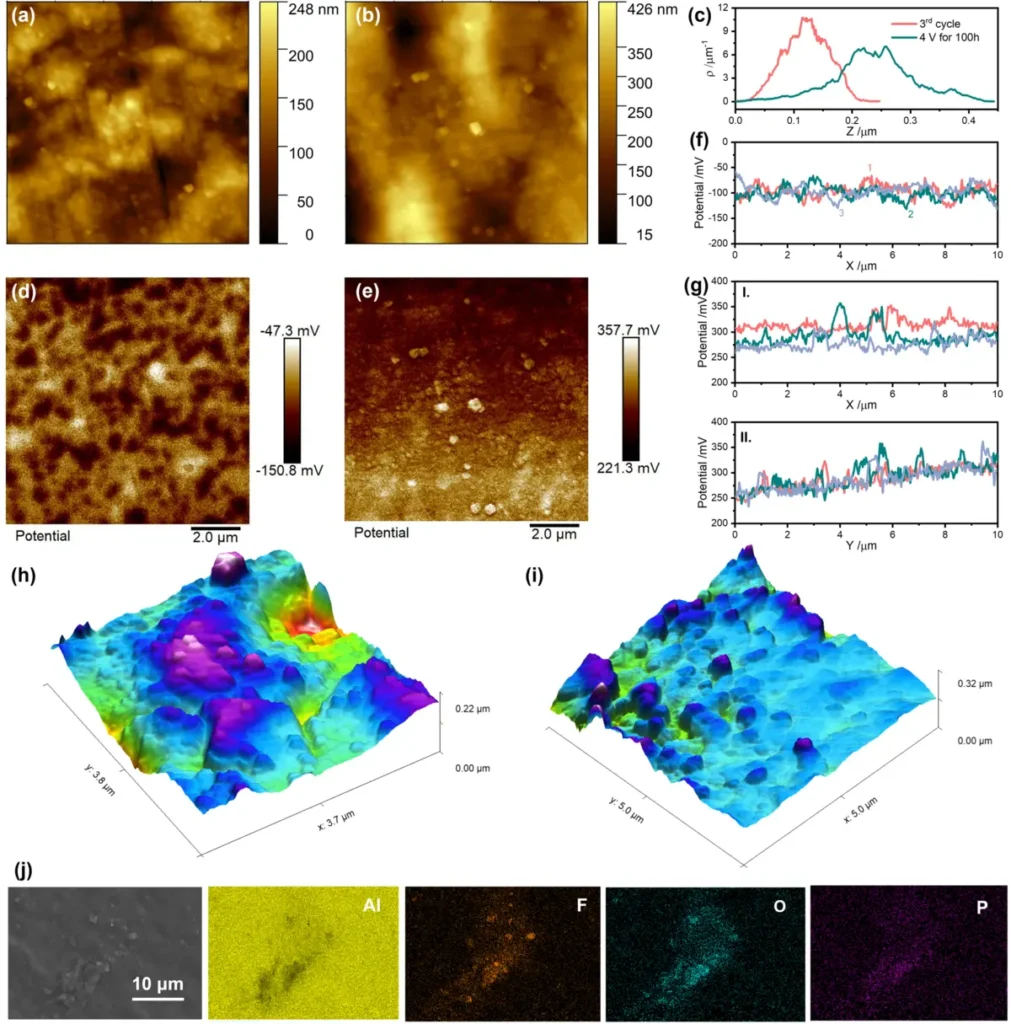
Analysis of specific instances and real-world implementations:
Multiple companies and research organizations are currently involved in the development of aluminum current collectors that are resistant to corrosion, to use them in commercial applications.
Real-World Examples:
Illustrations from the real-world Business organization. A has devised an exclusive technique for treating surfaces that greatly enhances the ability of Al current collectors to withstand corrosion. As a result, electric vehicle batteries have extended lifespans.
Research Institute B has been at the forefront of using nanostructured aluminum alloys as current collectors, demonstrating exceptional durability and efficiency in high-voltage lithium-ion batteries designed for large-scale energy storage on the power grid.
Implications for the environment and economy:
The widespread use of Li-ion batteries raises concerns about the environmental and economic ramifications of passivation and corrosion problems.
a). Issues relating to sustainability:
The exhaustion of resources and the creation of dangerous waste linked to battery production and disposal emphasize the significance of developing more sustainable and eco-friendly battery technologies.
b). There are factors to consider when determining the cost:
Consumers and organizations may suffer substantial economic losses due to the necessity for frequent battery replacements caused by passivation and corrosion-related problems. This emphasizes the significance of enhancing battery durability and reliability.
Obstacles and Constraints:
Despite significant progress in reducing passivation and corrosion, several obstacles and constraints remain that require attention.
a). Technological obstacles:
Attaining a state of equilibrium between passivation and conductivity is a significant obstacle, as too-thick oxide layers might hinder the movement of electrons.
Ensuring scalability and cost-effectiveness requires compatibility with existing battery manufacturing techniques and materials.
b). The market has limitations:
The battery industry’s competitive structure and the push to lower costs may prevent the widespread adoption of novel passivation and corrosion-resistant technology.
The article discusses the best practices for Al Current Collector maintenance:
Proactive maintenance and monitoring of Al current collectors are vital to ensuring optimal battery performance and durability.
a). Preventive Measures:
Regularly check for indicators of corrosion or passivation buildup.
Implementing suitable storage and handling methods to prevent exposure to corrosive conditions
b). Maintenance Protocols:
Periodic washing and surface treatment to eliminate pollutants and restore conductivity
To prevent further degradation, promptly replace corroded or damaged current collectors. The evolution of passivation layers on Al substrate.
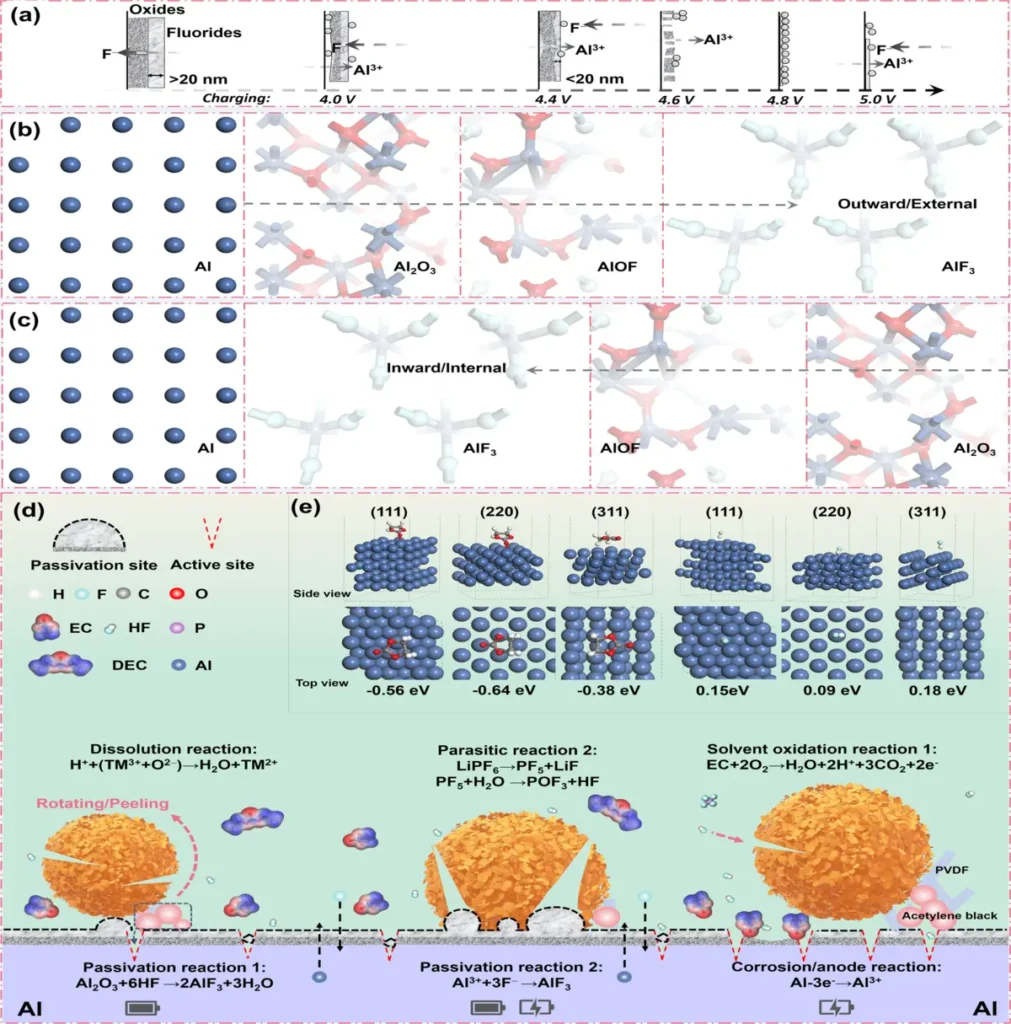
Collaborative efforts and industry standards:
Collaborative activities including industry stakeholders, research institutions, and regulatory agencies are vital to successfully handling passivation and corrosion concerns.
a). Regulatory Frameworks:
Standards and guidelines for the characterization and testing of corrosion-resistant materials and coatings have been developed.
The implementation of rules aims to promote the use of ecologically sustainable battery technology.
b). Collaborative Initiatives:
Public-private partnerships to fund research and development projects aiming at enhancing battery materials and manufacturing processes
Industry consortiums centered on pre-competitive collaboration and knowledge sharing.
Case Studies and Success Stories:
Several corporations and academic organizations have made great breakthroughs in creating corrosion-resistant Al current collectors.
a). Companies Leading in Corrosion-Resistant Technologies:
Company X has developed a unique surface coating that demonstrates remarkable corrosion resistance and endurance in tough working conditions.
Research Institute Y has found a novel alloy composition that optimizes the passivation capabilities of Al current collectors, leading to increased battery performance and longevity.
b). Notable research breakthroughs:
Researchers at University Z have pioneered the application of machine learning algorithms to anticipate the corrosion behavior of Al current collectors, enabling more accurate assessment and optimization of corrosion prevention techniques.
Future outlook and trends:
The future of passivation and corrosion prevention in Al current collectors seems optimistic, with continued improvements in materials research, surface engineering, and battery technology.
Emerging Technologies:
1). Integration of sophisticated nanomaterials and coatings to enhance corrosion resistance and conductivity
2). Development of next-generation Li-ion batteries with increased performance and endurance for diverse applications
Market Projections:
1). Continued growth in the demand for Li-ion batteries across the automotive, electronics, and energy storage industries
2). Increasing research and development investments to overcome critical issues and create new opportunities in battery technology.
Conclusion:
In conclusion, passivation and corrosion are significant elements that influence the performance and dependability of Al current collectors in Li-ion batteries. Understanding the underlying mechanisms and implementing appropriate mitigation techniques may help us overcome these obstacles and pave the way for more sustainable and resilient battery technologies.
FAQs:
1). Why are Al-current collectors prone to passivation and corrosion?
Aluminium easily reacts with oxygen and moisture in the environment, leading to the production of oxide layers that can either guard against further corrosion (passivation) or contribute to degradation (corrosion).
2). How do passivation and rust influence battery performance?
Passivation layers can make the electrode-electrolyte interface more resistant, which lowers conductivity and efficiency. Corrosion-induced degradation can cause capacity loss and shorter battery life.
3). What are some practical strategies to boost passivation and corrosion resistance?
Li-ion batteries routinely use surface treatments, alloying techniques, and nanostructuring to increase the stability and endurance of Al current collectors.
4). What are the environmental implications of battery passivation and corrosion?
Concerns regarding resource depletion and hazardous waste generation underline the significance of creating sustainable and environmentally friendly battery technology.
5). How might collaborative efforts and industry standards help overcome passivation and corrosion challenges?
By bringing together partners from business, academia, and government, collaborative projects can encourage innovation, establish best practices, and speed the adoption of corrosion-resistant battery solutions.
For more chemistry blogs, visit chemistry Master