Table of Contents
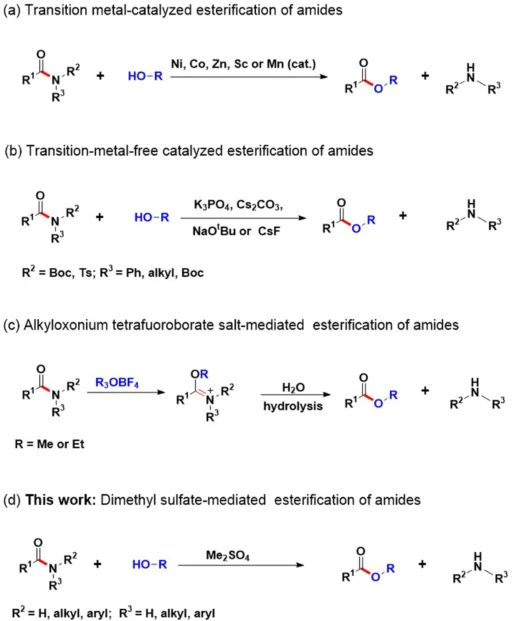
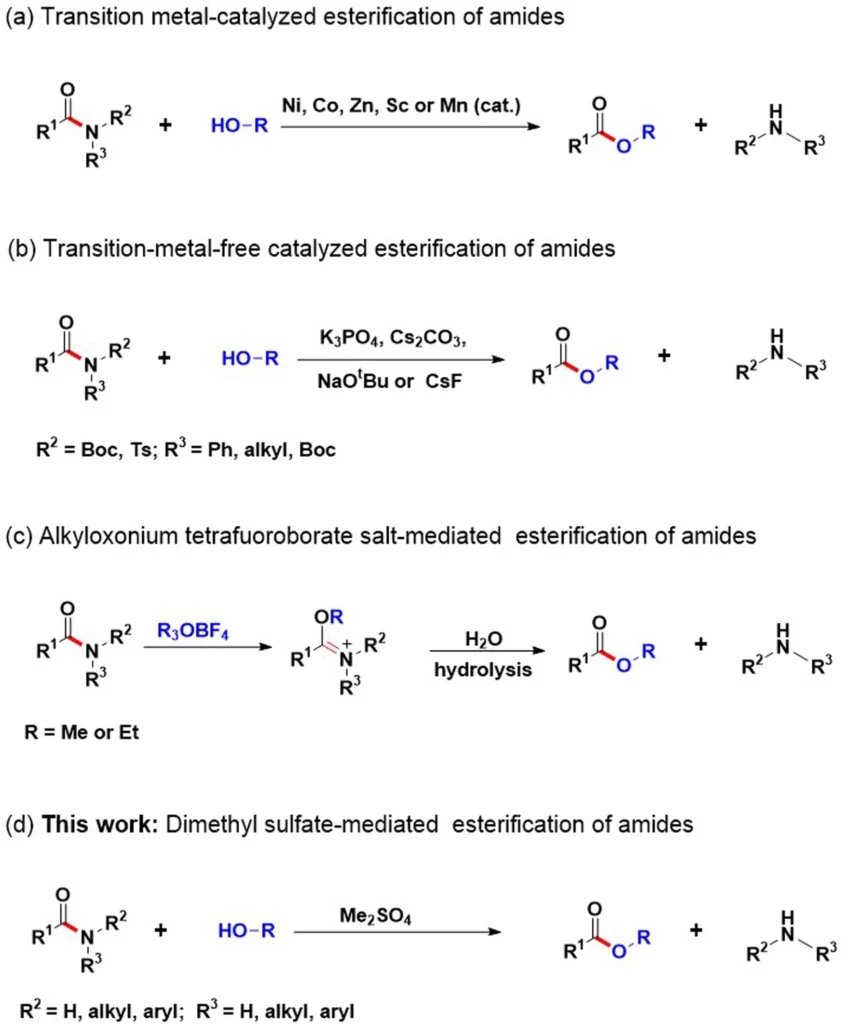
Amides are essential in organic chemistry since they are pivotal intermediates in various synthetic processes. Nevertheless, the inherent stability of these compounds presents difficulties when trying to achieve direct conversions, such as esterification. Conventional techniques frequently require severe conditions and multiple stages, which limit their effectiveness and suitability. Recently, researchers developed a new method that activates amide C-N bonds using dimethylsulfate (DMS). This strategy shows potential for addressing these difficulties.
An Overview of Amides and Amide Esterification:
Carboxylic acids and amines combine to form amides, which are chemical compounds. They are distinguished by a carbonyl group (C=O) connected to a nitrogen atom (NH2). Esterification is a crucial reaction in organic chemistry that involves the conversion of carboxylic acids into esters. This process allows for the incorporation of many chemical functionalities. Methods for amide esterification reactions.
Difficulties during the amide esterification process:
Resonance stabilization greatly reduces amide bonds’ reactivity to nucleophilic substitution processes, resulting in their high stability. The system’s intrinsic stability frequently necessitates the use of severe reaction conditions, such as high temperatures or strong acids, which may result in side reactions and limit the range of compatible substrates.
Dimethylsulfate activates amide C-N bonds:
Dimethylsulfate (DMS) is a chemical compound that acts as an electrophilic methylating agent. By adding a methyl group to the nitrogen atom, it enhances the reactivity of amide bonds. Methylation interferes with the amide’s ability to stabilize resonance, increasing its vulnerability to nucleophilic attack. Consequently, the amide can conduct esterification with alcohol under less severe conditions.
The mechanism involves the alcohol attacking the activated amide with its nucleophilic force, which makes an ester and dimethylamine a byproduct. DMS efficiently overcomes the inherent stability of amide bonds through activation, enabling direct conversions into esters.
The process of catalysis and the conditions for a chemical reaction:
Dimethyl sulfate (DMS) often facilitates the esterification process under mild conditions, negating the need for aggressive reagents. Nevertheless, the utilization of appropriate catalysts can greatly increase both the reaction rate and selectivity. People often use Lewis acids and Brnsted acids as catalysts to speed up reactions and expand the range of suitable substrates.
Scope and Applications of Amide Esterification:
You can use the DMS-mediated approach for a broad spectrum of amides, including primary, secondary, and tertiary derivatives. This adaptability provides opportunities for a wide range of uses in organic synthesis, medicinal chemistry, and materials research. This approach has produced compounds with ester functional groups that exhibit a wide range of characteristics and functions. Scope of alcohols for amide esterification.
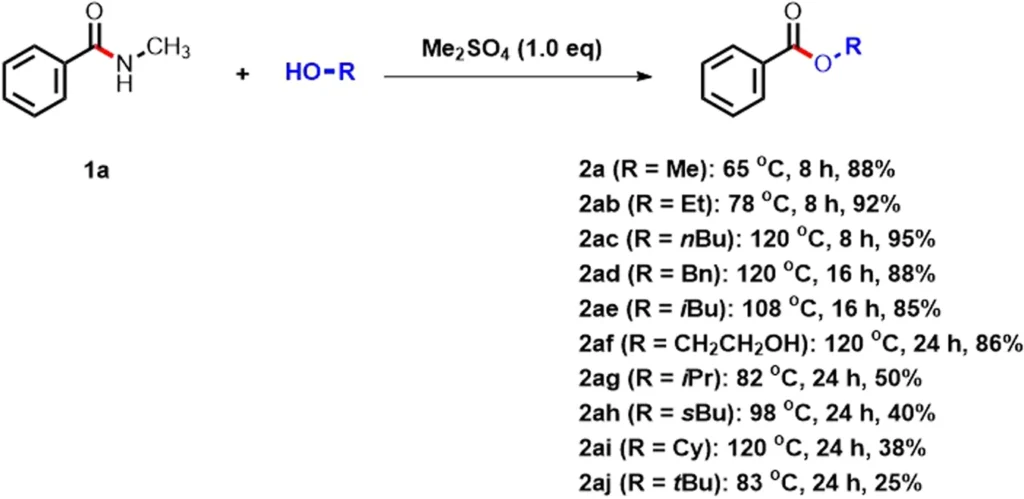
Yield-time curves of the amide esterification of 1a.
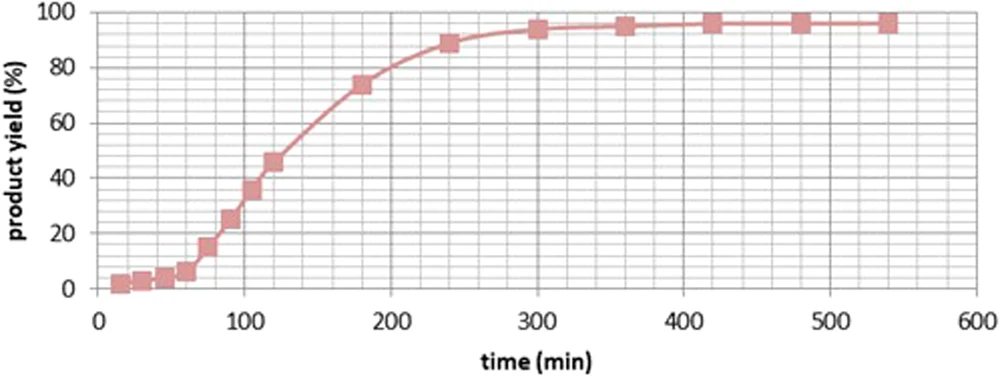
Comparative analysis with alternative methodologies:
DMS-mediated amide esterification has various benefits over previous methods that used strong acids or activated esters. It streamlines the process of creating synthetic pathways, minimizes the production of unwanted byproducts, and demonstrates enhanced selectivity. Furthermore, it reduces the use of hazardous substances, promoting environmentally friendly and long-lasting synthesis methods.
Current advancements and potential future outcomes:
The current research in this field aims to enhance the reaction conditions, explore novel catalysts, and expand the scope of amide structures under investigation. Recent advancements have showcased the capability of this approach to facilitate effective and feasible organic conversions. Potential future advancements include the creation of environmentally friendly and economically efficient methods for use in industrial settings. Control experiments and possible mechanisms.
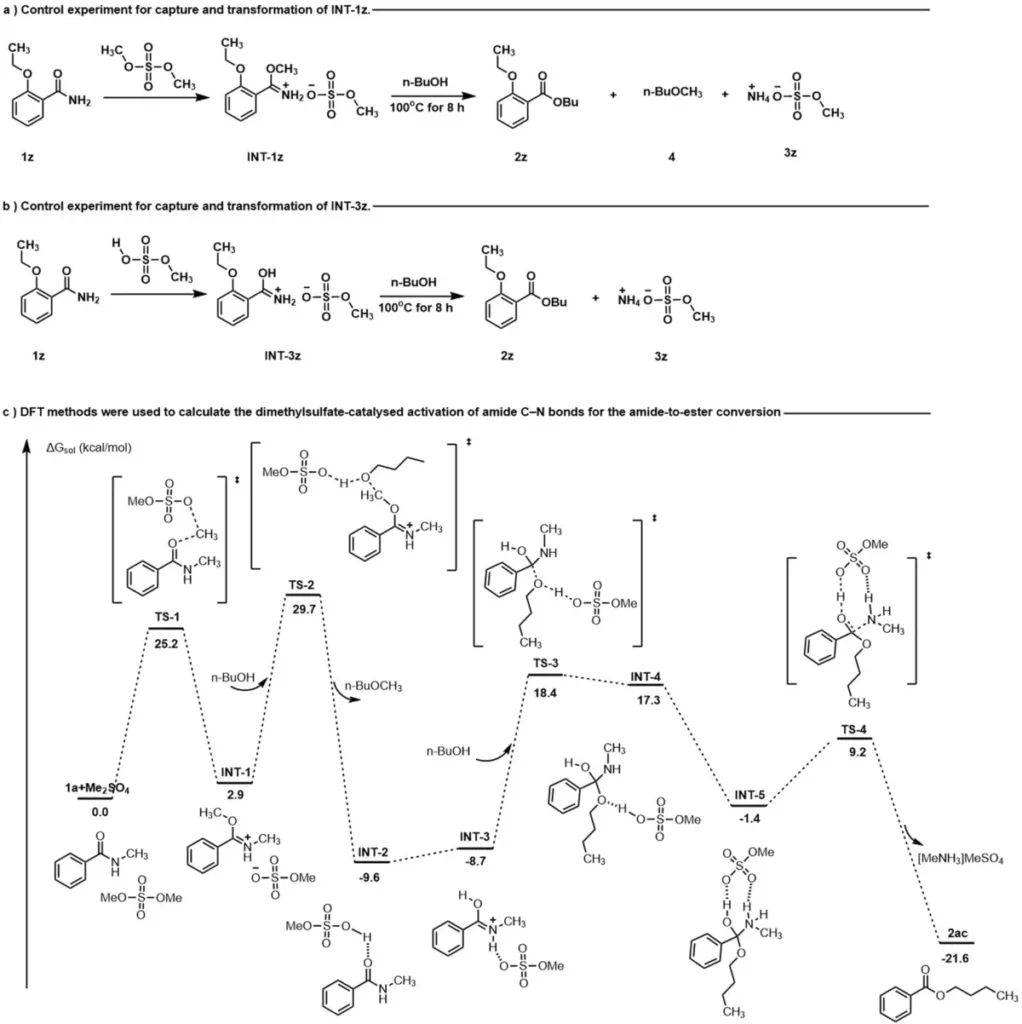
Factors that affect safety and the environment:
Although DMS-mediated esterification has notable benefits, it is crucial to take into account safety and environmental factors. Dimethylsulfate is a hazardous substance that necessitates cautious management and proper disposal. Scientists are now studying different substances and more environmentally friendly methods to reduce the possible harm to the environment.
In conclusion:
The process of directly esterifying amides by activating their C-N bonds with dimethylsulfate is a significant breakthrough in chemical synthesis. This novel method tackles enduring difficulties related to amide reactivity, opening up possibilities for enhanced, specific, and environmentally friendly chemical conversions.
Frequently Asked Questions:
1). What is the mechanism by which DMS activates amide bonds?
Dimethyl sulfate (DMS) adds a methyl group to the amide’s nitrogen atom. This breaks the amide’s resonance stability and makes it more reactive with nucleophiles like alcohol.
2). What are the advantages that direct amide esterification using DMS can offer?
Direct esterification simplifies synthetic processes, reduces the need for harsh chemicals, and expands the possible range of amide-derived molecules.
3). Which particular amide categories can DMS-mediated activation efficiently esterify?
You can esterify primary, secondary, and tertiary amides with DMS.
4). Does DMS-mediated esterification have a positive impact on the environment?
Although DMS-mediated esterification provides less harsh reaction conditions, the toxicity of DMS necessitates careful handling and proper disposal.
5). Are there any difficulties or obstacles in using DMS for the process of amide esterification?
Ongoing research focuses on the safety issues associated with DMS toxicity and the necessity of optimizing complicated amide substrates.
For more chemistry blogs, visit chemistry Master