Table of Contents
Opening statement:
Enantioconvergent synthesis is a very useful method in organic chemistry, especially for making chiral compounds that are very pure in terms of their enantiomeric makeup. This method is critical for the synthesis of chemicals used in a wide range of applications, from pharmaceuticals to cutting-edge materials. Axially chiral amides are particularly important because of their distinct structural characteristics and potential uses in medication development and catalysis.
Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation is a highly effective approach to producing axially chiral amides by utilizing Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation. This technology enables the efficient synthesis of complicated compounds and utilizes the enantioconvergent strategy to overcome the difficulties associated with conventional synthetic methods. This paper will investigate the complexities of this process, including the pivotal role of palladium catalysis in dynamic kinetic resolution and the transformative impact of this approach in the synthesis of axially chiral amides.
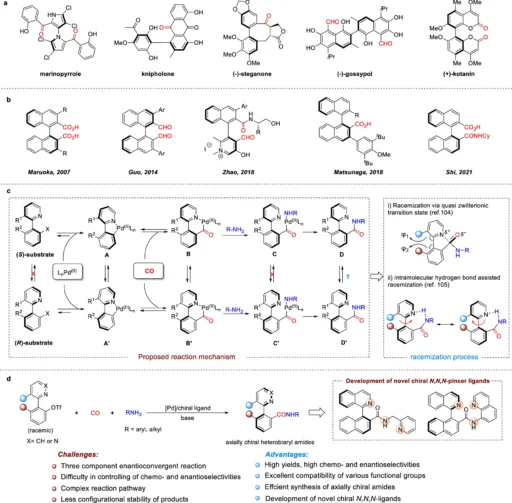
Comprehending Axial Chirality:
Axial chirality is distinct from point chirality because it originates from the spatial configuration around an axis rather than a single chiral center. Biaryl compounds, allenes, and certain amides commonly exhibit this type of chirality. This specific form of chirality holds significance as it yields unique chemical properties that find application in asymmetric catalysis, pharmaceutical research, and materials science. An important reason why axially chiral amides are so popular is that they could be used in enantioselective synthesis as ligands, catalysts, or basic building blocks in complex organic compounds. The importance of axially chiral carbonyl compounds and our design.
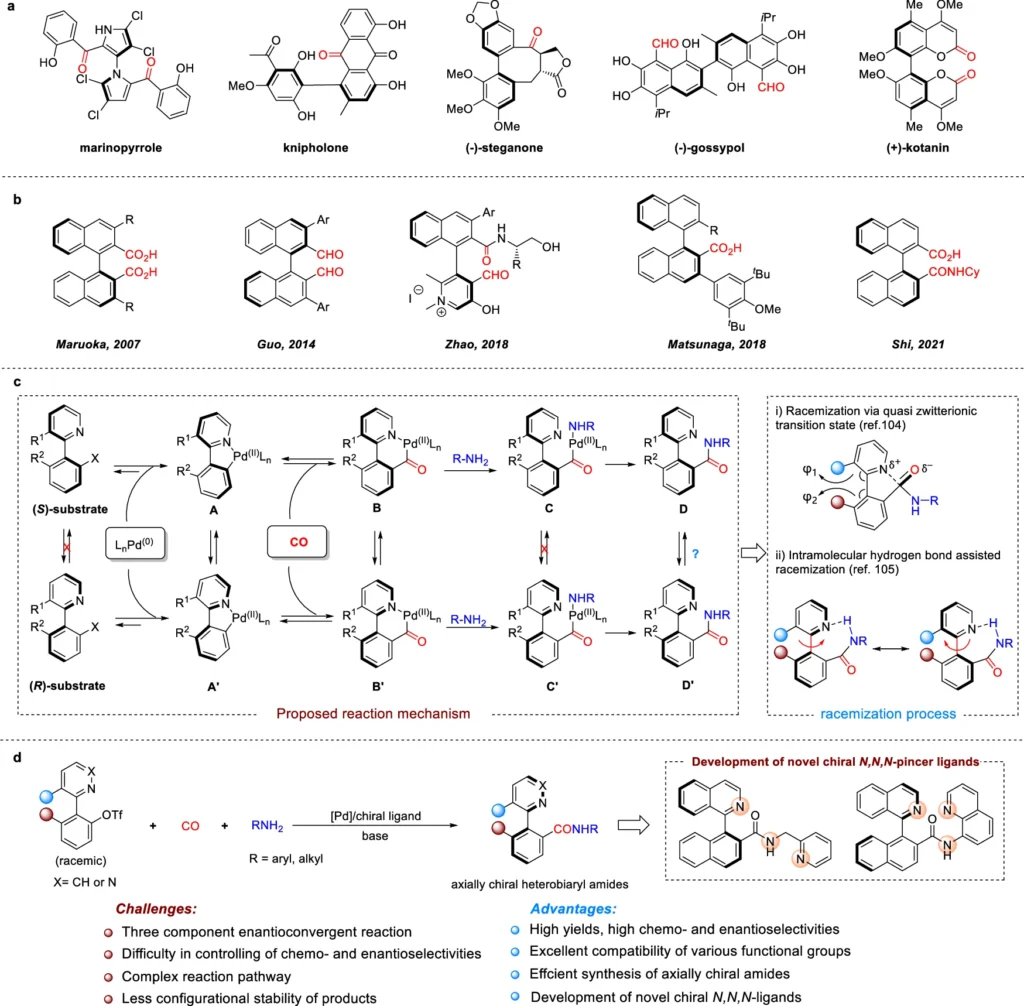
The process of synthesizing axially chiral amides is difficult:
Creating axially chiral amides is a challenging task. A low level of enantiomeric excess, the need for chiral starting materials, and the difficulty of controlling axial chirality during the process are all problems that are common with traditional methods. The constraints associated with axially chiral amides have hindered their widespread application in different industries, despite their considerable potential. Hence, there is a strong need for the development of a technique that enables the effective and selective production of these substances.
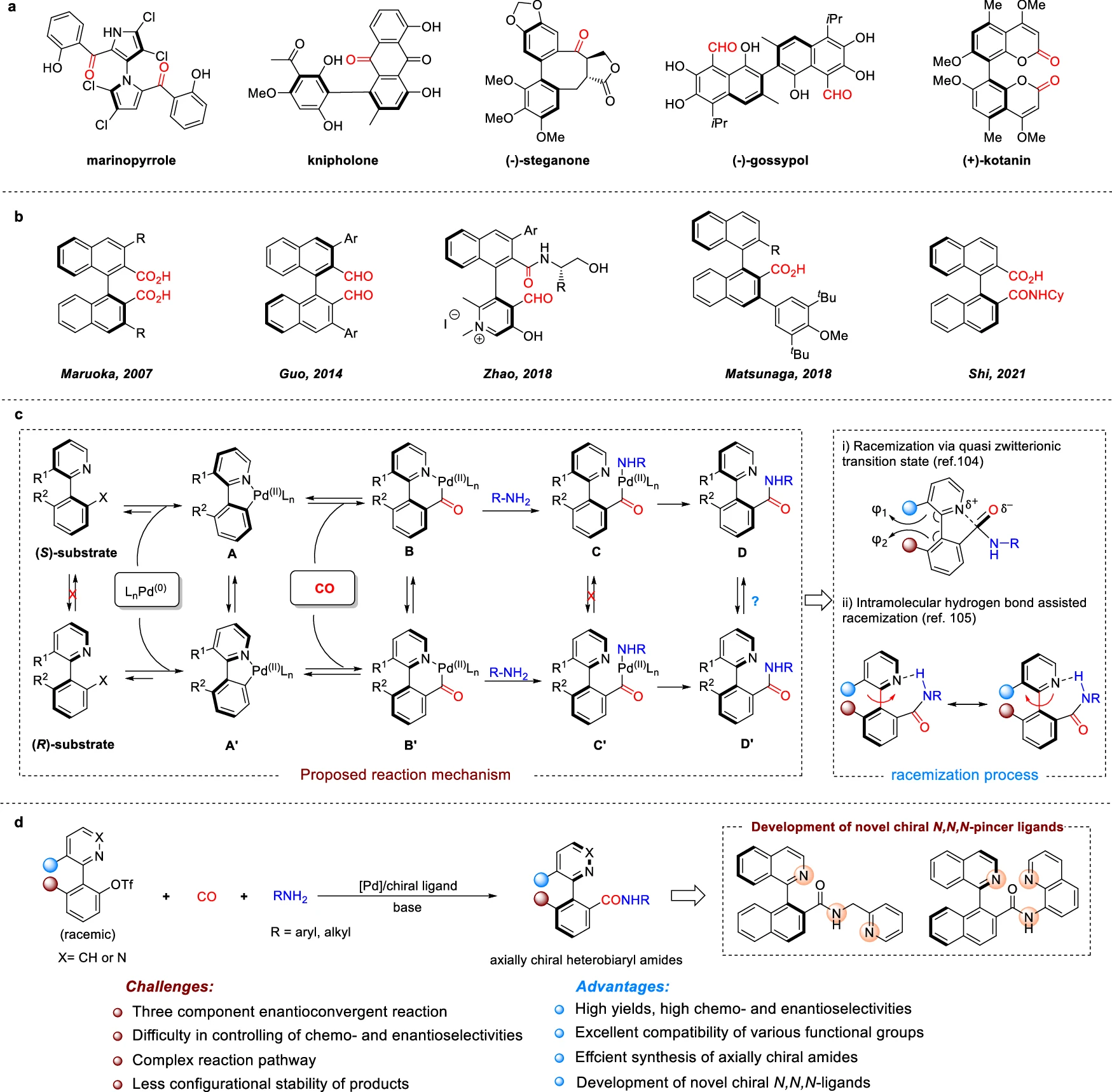
An Introduction to Enantioconvergent Synthesis:
Enantioconvergent synthesis is a method that enables the transformation of a racemic mixture into a solitary enantiomeric product. This is especially beneficial when the initial components are only available as mixtures of both enantiomers or when it is difficult to directly produce a single enantiomer from the starting materials. Enantioconvergent synthesis is distinguished by its ability to direct both enantiomers of the initial substance towards the formation of a single enantiomer of the end product, thereby optimizing both the quantity and purity of the enantiomer.
Role of Pd-Catalysis in Asymmetric Synthesis:
The role of Pd-catalysis in asymmetric synthesis is significant. Pd is a very flexible catalyst that is used a lot in organic synthesis because it can help with many different reactions, including cross-coupling, hydrogenation, and carbonylation. Pd-catalysis is very helpful in asymmetric synthesis because it can add chirality to the end product by reacting with chiral substrates or ligands. Dynamic kinetic resolution employs Palladium (Pd) to enhance its effectiveness, enabling the specific conversion of racemic mixtures into products with a high degree of enantioenrichment. Kinetic studies.
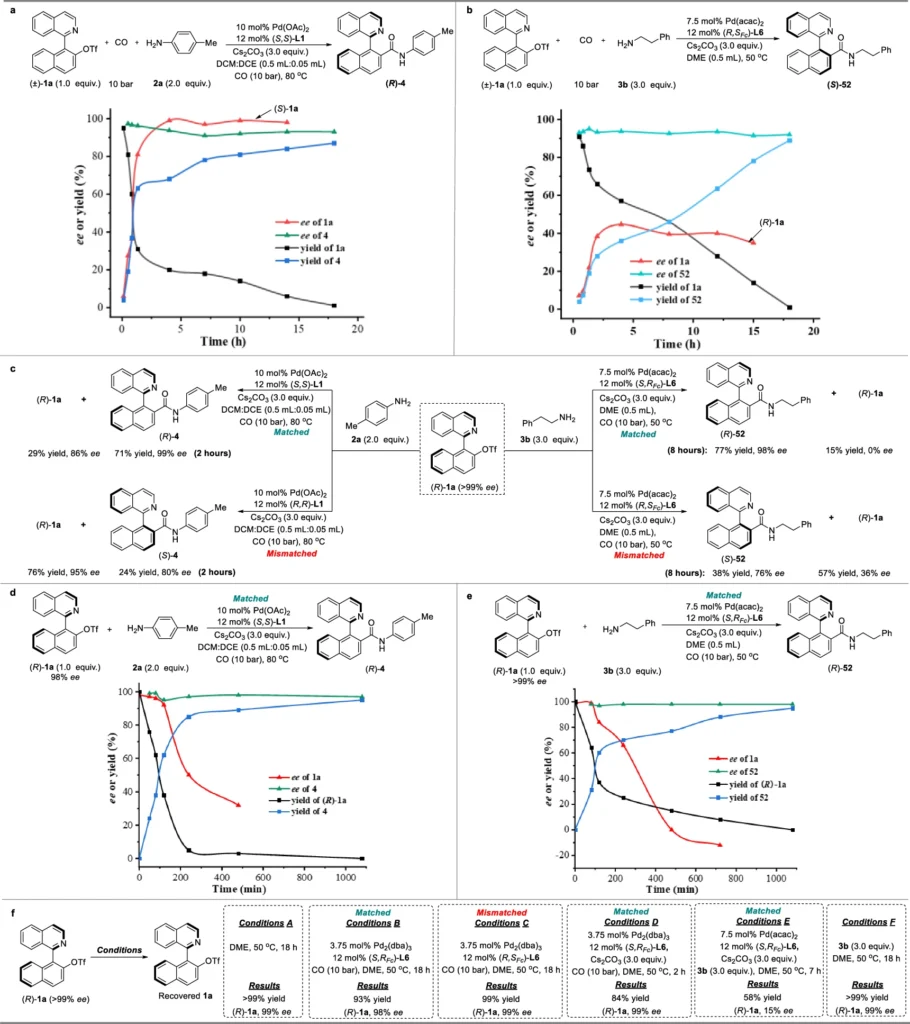
Dynamic Kinetic Resolution (DKR):
A process known as Dynamic Kinetic Resolution (DKR) selectively converts a mixture of enantiomers into a single enantiomer while also producing a racemic mixture of the other enantiomer.
Dynamic Kinetic Resolution (DKR) is a method that combines the processes of kinetic resolution with racemization, allowing the transformation of both enantiomers of a racemic mixture into a single enantiomeric product. We accomplish this by combining a rapid racemization process with a discriminating reaction that promotes one enantiomer. DKR is critical in Pd-catalyzed aminocarbonylation because it ensures that both substrate enantiomers are involved in the synthesis of the required axially chiral amide.
The Mechanism of Pd-Catalyzed Dynamic Kinetic Asymmetric:
Aminocarbonylation: This process is known as Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation.
The Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation process has several important steps that work together to make axially chiral amides with a lot of extra enantiomers.
Coordination and Activation: The Pd catalyst forms a coordination complex with the substrate, thereby activating it for subsequent reactions. Chiral ligands play a crucial role in promoting the desired chirality throughout this process.
Racemization: Racemization occurs when the substrate undergoes rapid interconversion between both enantiomers. This is critical for facilitating DKR, as it allows the less preferred enantiomer to undergo conversion into the more preferred one throughout the reaction.
Aminocarbonylation: An amide bond is made when a reactive substrate mixes with carbon monoxide (CO) and an amine. This is called aminocarbonylation. The process is extremely specific, as the Pd catalyst creates a chiral environment that guarantees the formation of the product with the necessary axial chirality.
Product Formation: The final product, an axially chiral amide, is formed with a high level of purity in terms of its enantiomers. Dynamic kinetic resolution (DKR) and Pd-catalyzed asymmetric synthesis work together to achieve this. Racemization experiment of (R)−4.
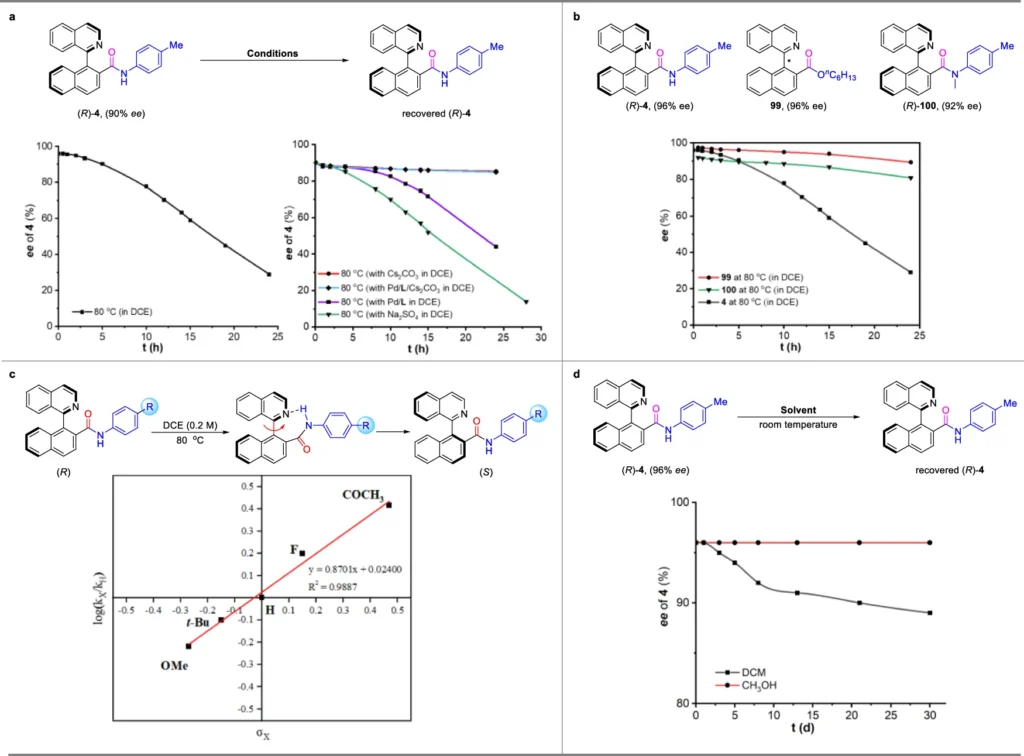
Aminocarbonylation is a critical step in synthesis:
Aminocarbonylation, a crucial process in amide production, involves inserting carbon monoxide (CO) into a palladium-amine complex to form the amide bond. This reaction has outstanding versatility, enabling the synthesis of a diverse array of amides under gentle reaction conditions. Anionic amides have axially chiral structures, and aminocarbonylation sets up the amide bond and controls the axial chirality. Consequently, it plays a crucial role in a comprehensive synthetic approach.
Enantioconvergence in the Reaction:
The phenomenon known as enantioconvergence occurs when two enantiomers, or mirror-image molecules, converge to form a single product in a reaction.
The Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation achieves enantioconvergence by allowing both enantiomers of the initial substance to participate in the creation of the desired product. The fast racemization of the substrate facilitates this phenomenon by ensuring the transformation of any surplus of one enantiomer into the other, leading to the exclusive creation of a single enantiomeric product.
Optimizing the conditions for a reaction:
How well and precisely the Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation process works depends a lot on the conditions in which it happens. Important factors to consider are:
Solvent: The choice of solvent can have a significant impact on both reaction rate and selectivity. Polar solvents often increase the solubility of carbon monoxide and amines, facilitating the aminocarbonylation process.
Ligands: Ligands, particularly chiral ligands, play a critical role in inducing the desired chirality in the product. By adjusting the steric and electrical characteristics of these ligands, it is possible to enhance enantioselectivity.
Temperature and Pressure: Elevated temperatures can expedite the racemization process, whereas the presence of CO can impact the rate of aminocarbonylation.
The analysis focuses on specific instances and recent progress:
New research shows several examples of how Pd can help with dynamic kinetic asymmetric aminocarbonylation to make axially chiral amides. Research has demonstrated the ability to synthesize axially chiral biaryl amides with high enantioselectivity, highlighting the potential of this approach in the synthesis of complex chiral compounds. These advancements highlight the increasing significance of Pd-catalysis in asymmetric synthesis. Scale-up synthesis of axially chiral amides and their applications in enantioselective catalysis.
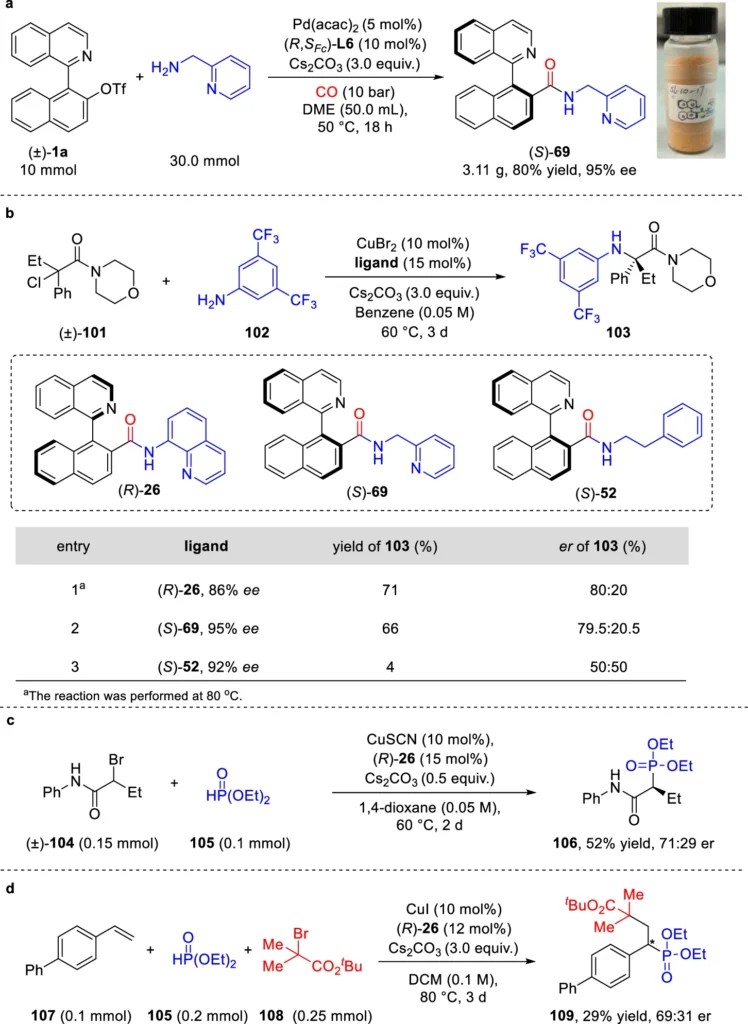
Utilization of Axially Chiral Amides:
Axially chiral amides have diverse applications in several fields. Drug development uses chiral ligands and catalysts to facilitate enantioselective synthesis, enabling the creation of medicines with high enantiomeric purity. Materials research uses axially chiral amides to create innovative polymers and liquid crystals with unique optical and electrical properties. Furthermore, their involvement in asymmetric catalysis is continuously growing, presenting fresh opportunities in the development of chiral catalysts.
The Pd-Catalyzed Method presents both pros and cons:
Pd-catalysis helps make axially chiral amides because it is highly selective for enantiomers, the reaction conditions are mild, and it can turn racemic mixtures into enantiomerically pure products. Still, there are some things to think about, like the fact that chiral ligands are expensive and there may be side reactions that lower both yield and selectivity.
Prospects for Future Advancements in Enantioconvergent Synthesis:
Advancements in enantioconvergent synthesis are driving the development of novel methodologies and catalysts that improve the efficiency and range of these reactions. New developments include using long-lasting catalysts, such as those made from metals that are easy to get, and looking into different reaction conditions that use less harmful solvents and higher pressures. We expect the continuous progress in Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation to significantly influence these breakthroughs, offering new opportunities for the production of intricate chiral compounds.
In conclusion:
The Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation makes it possible to make axially chiral amides, which is a big step forward in the field of asymmetric synthesis. Using the unique properties of palladium catalysis and the ideas of dynamic kinetic resolution, this technology makes it possible to make valuable chemicals quickly and selectively. As research advances in this field, we anticipate the emergence of other breakthroughs that will broaden the uses and availability of axially chiral amides, resulting in new opportunities in medicine development, materials science, and other areas.
Frequently Asked Questions:
1). A chemical process known as enantioconvergent synthesis converts a mixture of two enantiomers (mirror-image isomers) into a single enantiomer.
Enantioconvergent synthesis is a way to turn a racemic mixture into a single enantiomeric product, making the amount and purity of the enantiomer as high as possible.
2). What is the mechanism by which Pd-catalysis promotes asymmetric synthesis?
Palladium-catalysis makes it possible to make molecules that are rich in both enantiomers by using chiral ligands or substrates that make the final product chiral.
3). What advantages does dynamic kinetic resolution offer?
Dynamic kinetic resolution enables the preferential transformation of both enantiomers of a racemic mixture into a single enantiomeric product, thereby improving the overall yield and selectivity.
4). What is the significance of axially chiral amides?
Axially chiral amides are important because of their distinct structural characteristics, which make them useful in drug research, materials science, and asymmetric catalysis.
5). What are the upcoming challenges in this domain?
Upcoming difficulties include the development of more environmentally friendly catalysts, a reduction in reaction costs, and the extension of the approach to a wider variety of substances and chemical reactions.
For more chemistry blogs, visit chemistry Master