Table of Contents
Analytical Overview of Asymmetric Dihydroboration of Allenes:
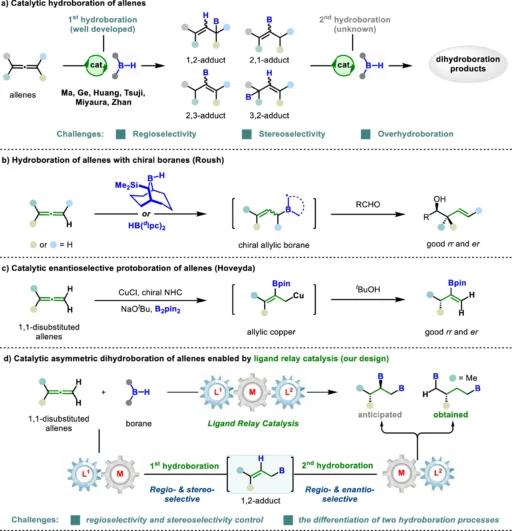
In contemporary organic chemistry, asymmetric dihydroboration has been a potent method for synthesizing significantly enantioenriched molecules. Boron hydrides can be carefully added to unsaturated compounds such as alkenes, alkynes, and, most recently, allenes using this method. When combined with the new ligand relay catalysis method, the dihydroboration process has reached levels of effectiveness and specificity that have never been seen before. Furthermore, what precisely is asymmetric dihydroboration, and how does ligand relay catalysis contribute to this process
What are allenes?
Allenes are organic molecules characterized by the presence of two neighboring carbon-carbon double bonds (C=C=C). What renders them especially captivating is their distinctive geometry—an allene molecule assumes a linear configuration with orthogonal π-systems, which imparts it with fascinating reactivity. These compounds are very adaptable intermediates in the process of organic synthesis and function as fundamental components for a diverse range of natural goods, medicines, and materials. Hydroboration of allenes.
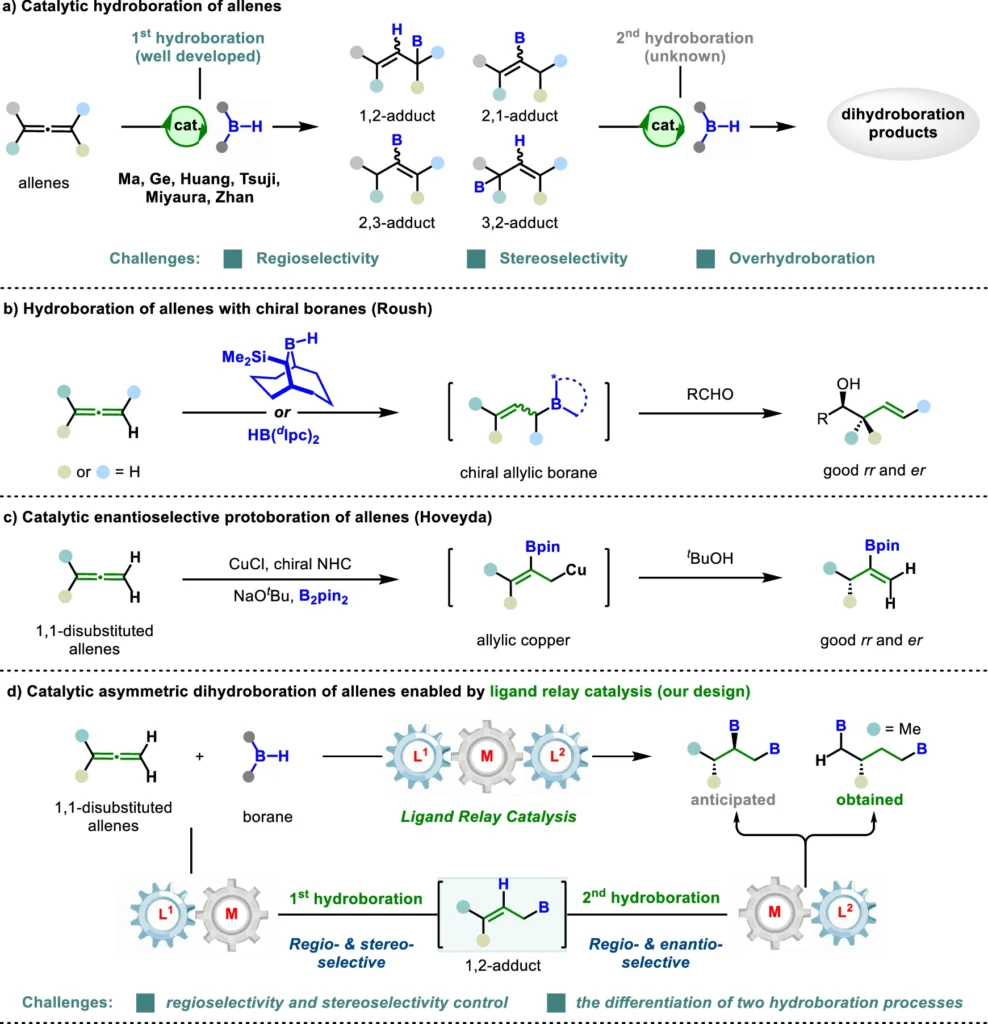
The Dihydroboration Concept:
Adding a boron-hydrogen (B-H) bond across unsaturated carbon-carbon bonds, like those found in alkenes or allenes, is what dihydroboration is all about. The reaction yields organoboron compounds, which serve as important intermediates for further chemical conversions, such as cross-coupling reactions. The goal of asymmetric dihydroboration is to achieve a significant degree of enantioselectivity, resulting in products with highly defined three-dimensional structures—essential for several applications in medicine and fine chemicals.
The Significance of Asymmetric Dihydroboration:
In contemporary synthetic chemistry, enantioselectivity is a fundamental aspect, particularly in the field of pharmaceuticals, where the spatial configuration of atoms can impact the effectiveness, strength, and safety of a therapeutic agent. Asymmetric dihydroboration enables chemists to precisely induce chiral compounds, ensuring that only one enantiomer (out of two potential mirror-image forms) is produced in surplus. Enantiomers of a medicine can have significantly different biological effects, making this matter very important. Scope of allenes.
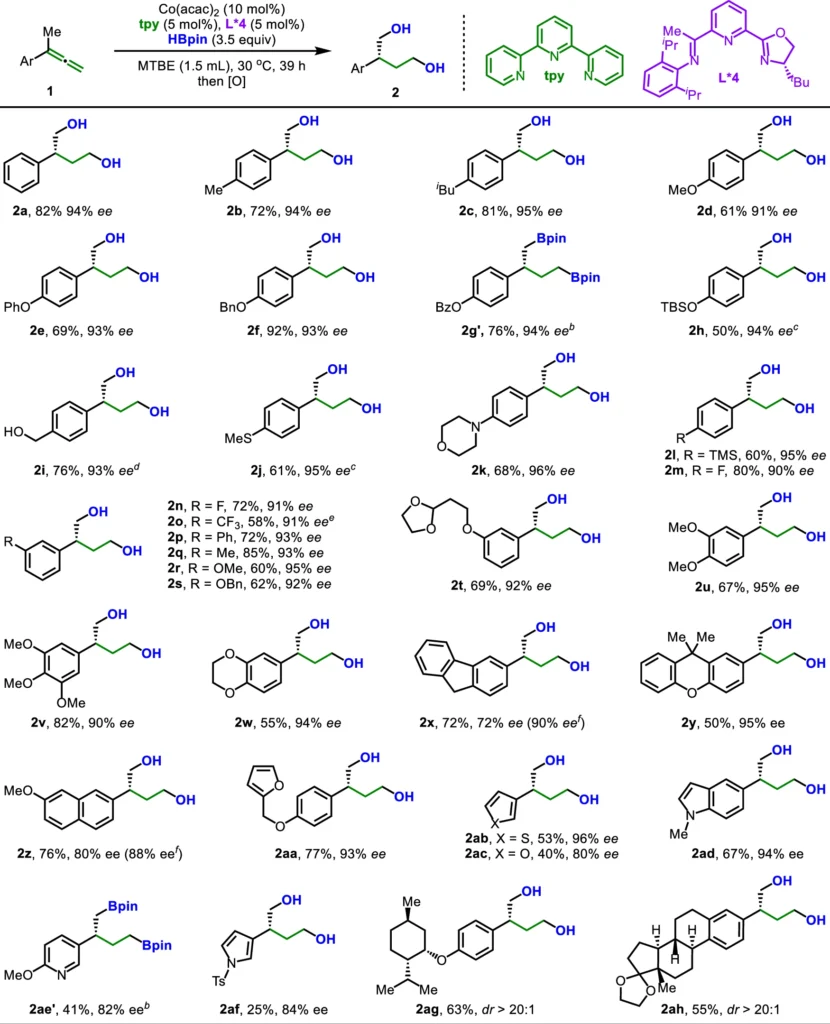
An Overview of Ligand Relay Catalysis:
The concept of ligand relay catalysis is a recently developed approach in catalysis that facilitates more precise chemical reactions. In conventional catalysis, a solitary ligand forms a complex with a metal catalyst during the entire reaction. At various stages of the reaction, ligand relay catalysis involves the dynamic interaction of many ligands with the metal center. This phenomenon of the relay effect guarantees a precise adjustment of the reaction pathway, resulting in improved regulation of both selectivity and reactivity.
The Effect of Ligand Relay Catalysis on Dihydroboration:
The inclusion of ligand relay catalysis in asymmetric dihydroboration has numerous benefits. The process enables accurate activation and stability of intermediates, thereby aiding the enantioselective synthesis of the intended product. The dynamic nature of the ligand exchange bolsters the reaction’s controlled stereochemistry, ensuring the utilization of the allene’s distinctive geometry to produce enantiomerically pure products.
Limitations of Conventional Dihydroboration:
Before the invention of ligand relay catalysis, it was often hard to achieve a high level of enantioselectivity in dihydroboration. It was hard for traditional catalytic systems to control how the boron hydride addition rotated, especially when it came to allenes because the π-bonds are not parallel. Furthermore, conventional systems typically exhibited a lack of adaptability and required thorough fine-tuning for various substrates, thereby reducing their feasibility for broad implementation. Synthetic transformations and applications.
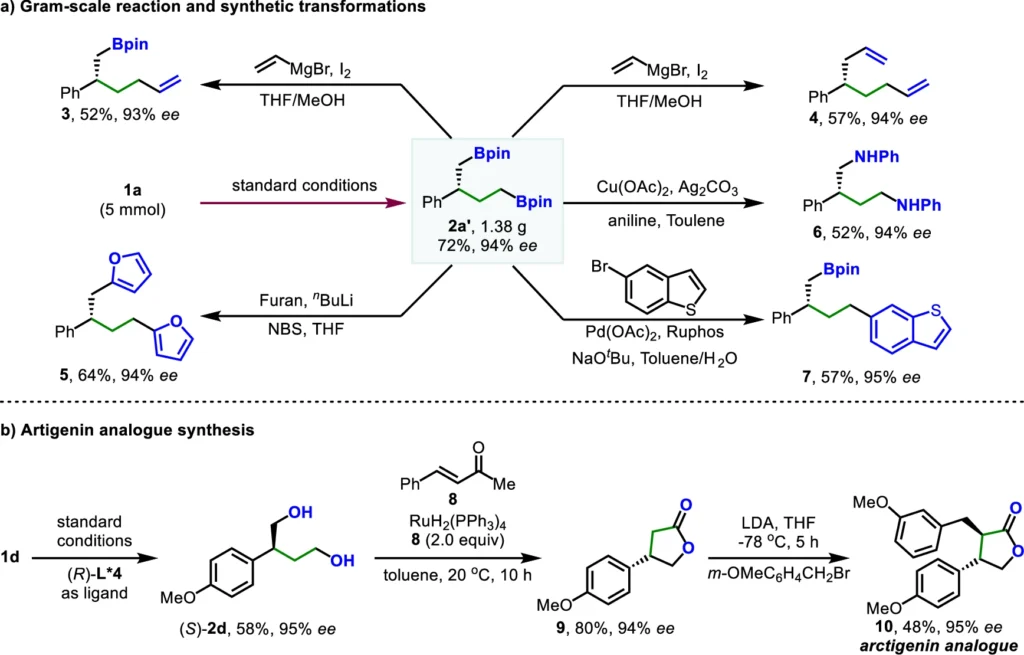
Asymmetric dihydroboration of allenes has several advantages:
The utilization of ligand relay catalysis for asymmetric dihydroboration of allenes presents several advantages:
High enantioselectivity: It exhibits high enantioselectivity, resulting in the selective production of a single enantiomer.
Mild reaction conditions: The mild reaction conditions allow for the conduction of these reactions at ambient temperature, hence enhancing their environmental friendliness.
Broader substrate scope: This approach’s broader substrate scope allows it to be used with a wide range of allene derivatives, enhancing its versatility in synthetic applications.
The operational mechanism of asymmetric dihydroboration of allenes is presented:
The first step in the ligand relay catalysis for asymmetric dihydroboration of allenes is for the allene atom to bind to a metal center complex. A ligand relay system ensures that appropriate ligands are present at critical points to direct the reaction through a controlled sequence of well-defined stages. Firstly, we carefully introduce the boron hydride to a specific double bond in the allene. Furthermore, a dynamic ligand exchange process stabilizes the intermediate, allowing for subsequent addition and final synthesis of the enantioenriched product. Mechanistic studies.
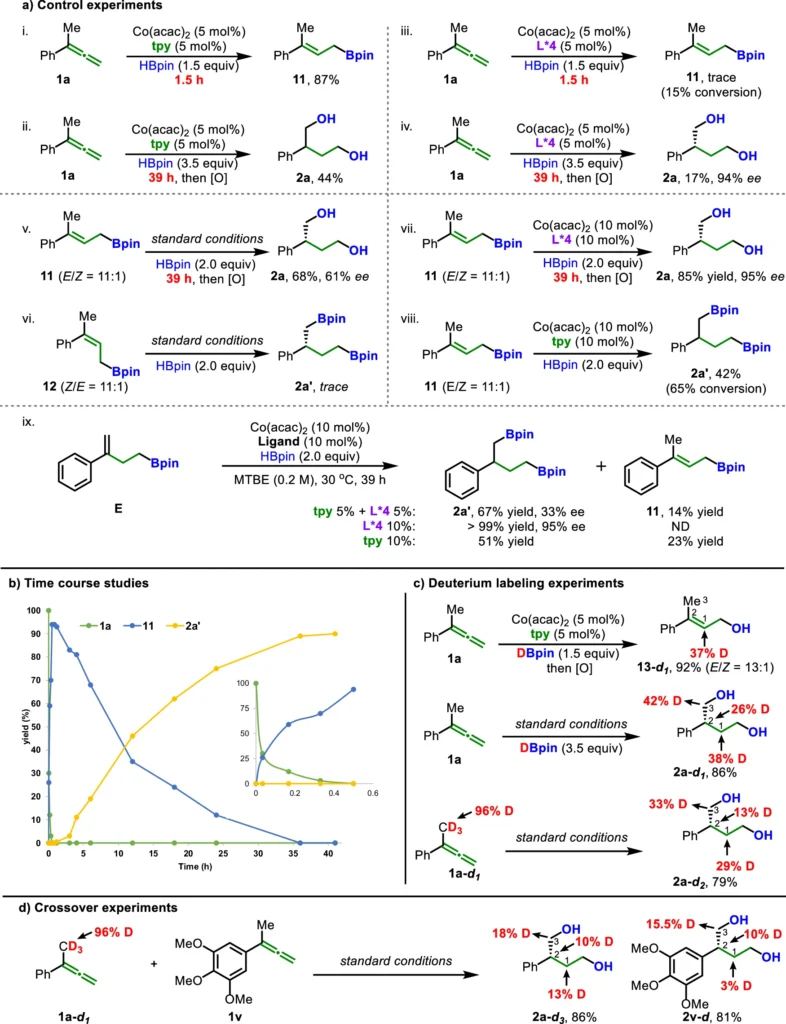
Ligand Relay Catalysis: An Examination of Ligands:
An essential function of ligands is to regulate the outcome of catalytic processes. Crucial in the realm of ligand relay catalysis is the meticulous design of ligands. Commonly used ligands include phosphone-based ligands, chiral ligands, and nitrogen-containing species. To improve high enantioselectivity, it is important to create ligands that not only keep the metal center stable but also work with the substrate in a way that makes this process easier.
Illustrations of Effective Responses:
Extensive research has demonstrated the high effectiveness of ligand relay catalysis in the asymmetric dihydroboration of allenes. Notably, reactions that include palladium or rhodium catalysts with chiral phosphine ligands have shown remarkable selectivity. These reactions often produce large quantities and typically yield enantiomerically pure products, making them ideal for further synthetic manipulation.
Asymmetric dihydroboration applications in synthesis:
Asymmetric dihydroboration produces adaptable intermediates that can transform into a wide range of useful compounds. These reactions, in particular, are critical for the production of complex natural compounds and pharmaceutical compounds. Moreover, the synthesis of agrochemicals and materials that require precise molecular structures has utilized these reactions.
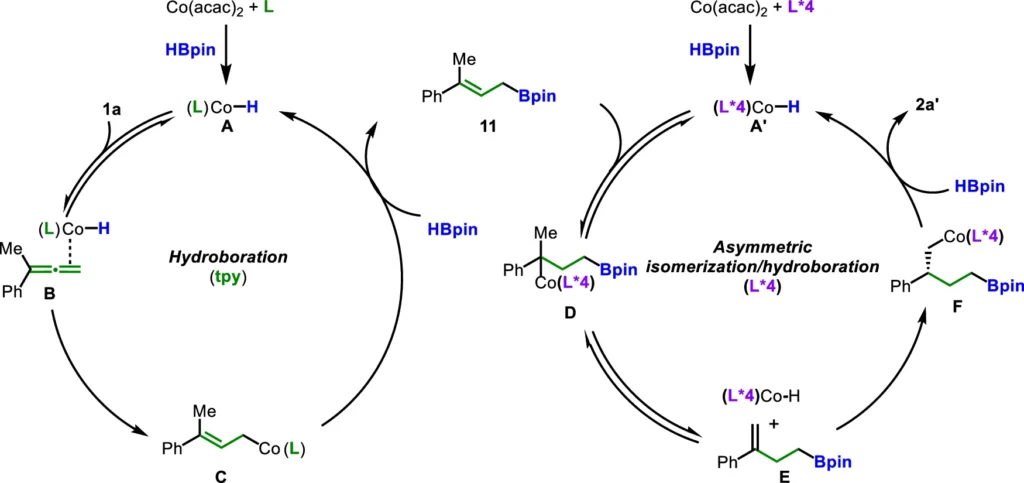
Cutting-edge developments in ligand relay catalysis:
The domain of ligand relay catalysis is undergoing rapid expansion. Recent advancements include the identification of novel ligand systems that allow for enhanced manipulation of reaction characteristics. Additionally, researchers are looking into whether this catalytic method could be used for other types of chemical changes, such as asymmetric hydrogenation and cross-coupling reactions.
The method presents several challenges and limitations:
Although the use of ligand relay catalysis for asymmetric dihydroboration has yielded impressive results, there are still unresolved issues that require attention. The approach may exhibit reduced efficiency for specific categories of allenes or when subjected to more critical reaction conditions. Furthermore, the process of designing and synthesizing appropriate ligands can be both time-consuming and expensive.
In conclusion:
A huge step forward in the field of organic synthesis is the use of ligand relay catalysis to achieve asymmetric dihydroboration of allenes. This approach offers a very specific and effective means of synthesizing enantiomerically pure molecules, which are essential in the advancement of medicines and fine chemical technologies. Through continuous investigation of ligand design and catalytic mechanisms, the future of this discipline appears to be highly promising, as possible applications are expanding into novel domains of synthetic chemistry.
Frequently Asked Questions:
1). What’s the significance of asymmetric dihydroboration in organic chemistry?
Chemists can synthesize compounds with a high enantiomerization through asymmetric dihydroboration, which is crucial for applications like pharmaceuticals where the three-dimensional arrangement of atoms determines the biological activity.
2). What effect does ligand relay catalysis have on reaction outcomes?
Ligand relay catalysis promotes dynamic ligand exchange, thereby enabling better regulation of the reaction pathway and boosting both reactivity and selectivity.
3). Can we extend ligand relay catalysis to include other chemical reactions?
Indeed, a wide range of asymmetric reactions, such as hydrogenation and cross-coupling, can benefit from this catalytic approach.
4). Asymmetric dihydroboration is subject to some constraints.
The approach may encounter difficulties with specific substrates and necessitate meticulous ligand design, which can be a computationally demanding process.
5). Asymmetric dihydroboration plays a significant role in the advancement of pharmaceutical development.
This technology facilitates the synthesis of chiral medicinal molecules with exceptional accuracy, a crucial factor in guaranteeing their effectiveness and safety.
For more chemistry blogs, visit chemistry Master