Table of Contents
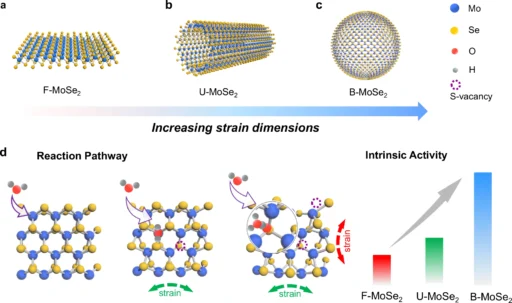
Overview of Biaxial strain:
The Hydrogen Evolution Reaction (HER) is essential for the production of hydrogen fuel, which is a clean and sustainable energy source that plays a crucial role in reducing reliance on fossil fuels. Kinetic problems specific to alkaline HER impede the efficiency of hydrogen production. This paper investigates the use of biaxial strain-induced OH engineering to improve the alkaline hydrogen evolution reaction’s (HER) rate. It investigates the principles, materials, synthesis methodologies, and prospects of this novel approach.
Hydrogen, as an environmentally friendly energy carrier, has the potential to be used in a wide range of applications, including fuel cells and industrial operations. Nevertheless, the task of achieving efficient hydrogen production continues to be a substantial obstacle. The HER (hydrogen evolution reaction) process, which entails the electrolysis of water to generate hydrogen and oxygen, is essential for hydrogen production. In alkaline conditions, the hydrogen evolution reaction (HER) moves more slowly than in acidic conditions because of extra energy barriers. This makes the reaction less efficient. This lack of efficiency poses a significant barrier to the general acceptance of hydrogen as a sustainable energy source. Definition and impact of zero strain, uniaxial strain, and biaxial strain.
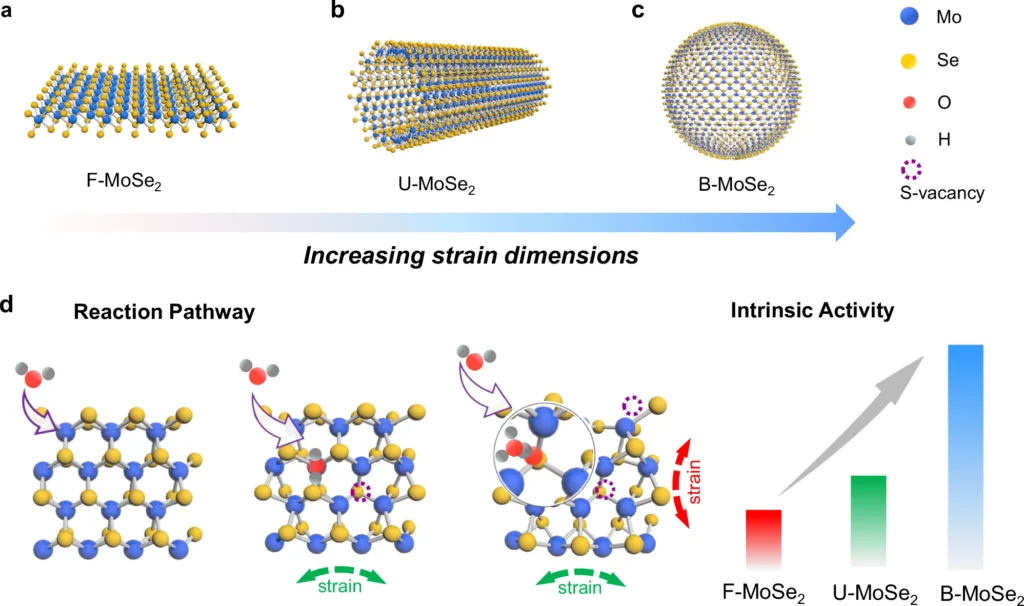
Biaxial Strain in Catalysis:
Biaxial strain in catalysis refers to the application of stress in two perpendicular directions on a catalyst material, which can significantly influence its catalytic activity. Biaxial strain, derived from materials science, denotes the concurrent imposition of stress in two mutually perpendicular directions. This strain can alter the lattice structure of materials, resulting in modifications to their electrical characteristics and catalytic behavior. Biaxial strain in catalysis can improve catalyst performance by modifying their surface characteristics, electrical configuration, and contact with reactants.
Catalysis has long employed strain engineering. Semiconductor technology has been employed to enhance the efficiency of electrical devices. Nevertheless, its utilization in catalysis, particularly for HER, has only emerged in recent times. Scientists have found that subjecting catalysts to biaxial strain can greatly improve their capacity to catalyze reactions, maintain stability, and exhibit selectivity.
Explanation of Biaxial Strain Mechanisms:
At the atomic level, biaxial strain has an impact on the interatomic distance and the distribution of electronic density within the material. As a result, this affects the locations where the HER catalytic process takes place. By changing the electrical properties, biaxial strain can make it easier for hydrogen intermediates to attach and speed up the reaction as a whole.
Applying strain to a material results in deformations in the atomic lattice. These distortions can alter the material’s electrical configuration, affecting the distribution of electrons and their interactions with adsorbed species. For HER, this implies that the stressed catalyst can more effectively stabilize hydrogen intermediates, thereby decreasing the energy barrier for the reaction and enhancing the total reaction rate.
An engineer specializing in Alkaline Hydrogen Evolution Reaction (HER):
Hydroxyl (OH) groups are essential in the process of hydrogen evolution reaction (HER), especially under alkaline circumstances. These groups serve as intermediaries in the process of transferring protons, aiding the conversion of water into hydrogen. Manipulating these hydroxyl (OH) groups via biaxial strain can greatly increase their chemical reactivity.
Efficient proton transfer in alkaline HER relies on the crucial presence of OH groups on the catalyst surface. Applying pressure to catalysts improves their ability to stabilize OH intermediates, allowing them to participate in the reaction. The total hydrogen evolution reaction (HER) rate can be sped up by a large amount because hydroxyl (OH) groups are more stable and reactive. Computation of the biaxial strain effect on water adsorption and hydrogen evolution.
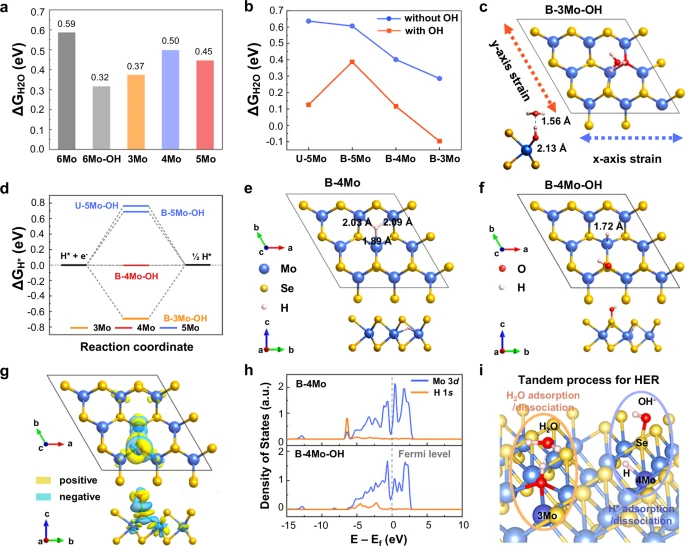
The materials for the engineering of OH via Biaxial Strain:
Transition metal oxides, nitrides, and carbides are effective materials for engineering OH by biaxial strain. These materials have the requisite structural characteristics to endure stress and demonstrate improved catalytic efficiency. Recent research has identified materials such as NiFe, CoP, and MoS2 as highly promising options.
Transition metal oxides such as nickel oxide (NiO) and cobalt oxide (CoO) are known for their stability and catalytic prowess. By applying biaxial strain to these materials, it is possible to adjust their electrical characteristics to improve their catalytic activity. Similarly, compounds classified as nitrides and carbides, such as molybdenum nitride (MoN) and tungsten carbide (WC), have shown significant promise in the field of strain engineering.
Production of Stressed Catalysts:
Catalysts can achieve biaxial strain through various methods, including epitaxial growth, thermal expansion mismatch, and mechanical deformation. Advanced techniques such as X-ray diffraction, electron microscopy, and Raman spectroscopy are necessary to characterize these strained materials. Optimization strategies aim to achieve a consistent distribution of strain and improve the stability of materials.
Epitaxial growth is a widely used method to induce biaxial strain in thin films. Growing a thin layer of material on a substrate with a variable lattice constant induces strain in the film. When we heat a material to high temperatures and then rapidly cool it, the disparity in thermal expansion coefficients causes strain, known as thermal expansion mismatch. Large quantities of materials can also experience strain due to mechanical deformation, such as bending or stretching.
Empirical Investigations and Findings:
Recent experimental studies have shown that biaxial strain catalysts have led to significant improvements in HER performance. For instance, strain-submitted NiFe catalysts exhibit a tenfold increase in activity compared to their unstrained counterparts. Performance parameters like overpotential, Tafel slopes, and exchange current density quantify these gains.
Experimental investigations frequently employ electrochemical measurements to assess the efficacy of strained catalysts. Overpotential, which refers to the additional voltage needed to initiate the reaction, is a crucial measure of the performance of the hydrogen evolution reaction (HER). Reduced overpotentials indicate that catalysts are more efficient. Tafel slopes, which establish the correlation between the overpotential and the current density, offer valuable information about the reaction kinetics. The exchange current density, which represents the current density at zero overpotential, is an important quantity that indicates the catalyst’s inherent activity.
Theoretical insights:
Computational models and simulations offer useful insights into the impact of biaxial strain on catalysts. These models forecast the impact of strain on electronic properties and catalytic activity, guiding the development of more efficient catalysts. Simulations are also useful for determining the most effective levels of strain and material composition.
We frequently employ density functional theory (DFT) computations to investigate the electrical structure of materials under tension. These computations help forecast the impact of strain on the energy levels and distribution of electrons within the material. Molecular dynamics (MD) simulations offer valuable insights into the atomic-level interactions and dynamics of catalysts under strain. By integrating Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations, researchers can get a holistic comprehension of the impact of strain on catalytic performance.
Benefits of Biaxial Strain Engineering:
Biaxial strain engineering offers a notable improvement in the kinetics of the hydrogen evolution reaction (HER). Under strain, catalysts exhibit reduced overpotentials and increased current densities, resulting in improved efficiency. Moreover, this approach can be economically advantageous as it utilizes pre-existing resources and manufacturing methods.
Biaxial strain engineering has numerous benefits compared to conventional approaches to enhancing HER (hydrogen evolution reaction) performance. Strain engineering can greatly improve the activity of catalysts by altering their electrical characteristics, eliminating the need for costly or scarce ingredients. This approach is cost-effective for developing catalysts with high performance. Alkaline HER performance.
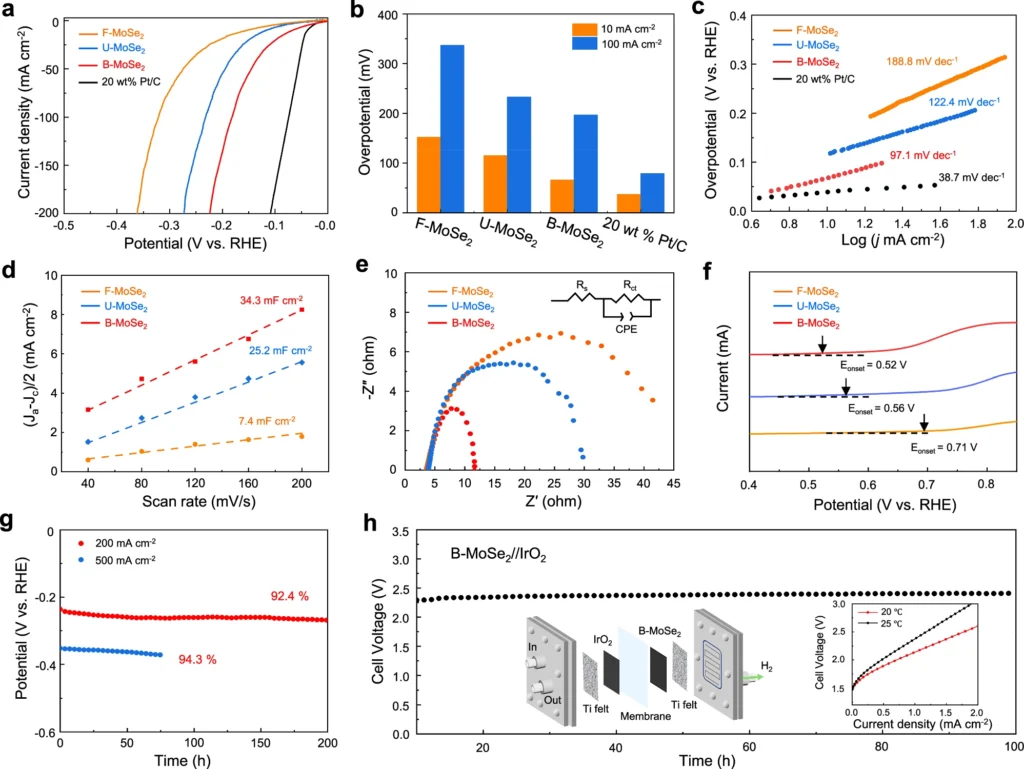
Obstacles and Constraints:
Although biaxial strain engineering holds promise, it encounters many obstacles. Technical challenges include achieving consistent strain, preserving material stability during operational conditions, and expanding the production process. The stability of materials is a crucial concern, as catalysts under duress may experience alterations in their structure or deterioration over a while.
Attaining consistent strain throughout the catalyst surface is difficult, particularly for materials in large quantities. Variations in strain distribution can cause inconsistent catalytic performance. Another issue to consider is the material’s stability, as strained materials have the potential to gradually relax over time, thereby reducing their efficiency. To increase the production of strained catalysts for industrial applications, certain technological obstacles must be overcome.
Prospects for the future:
Anticipated advancements in catalyst design will tackle these problems. This technology’s potential applications go beyond hydrogen production. Other electrochemical processes like the oxygen evolution reaction (OER) and the carbon dioxide reduction reaction (CO2RR) can also utilize this technology. Biaxial strain-influenced catalysts can enhance the economic and environmental benefits of renewable energy systems.
Continuous research and improvement in strain engineering are critical for fully realizing its maximum capabilities. The advancement of synthesis techniques, material characterization, and computational modeling will be the driving force behind the creation of strained catalysts that are both more effective and stable. Furthermore, the integration of these catalysts with renewable energy sources, such as solar and wind, might amplify their influence on sustainable energy generation.
Practical Implications:
Two-dimensional biaxial strain-induced engineering is a very important tool for industry because it lets us make hydrogen production more efficient and environmentally friendly. From an economic standpoint, this could potentially lower the costs associated with hydrogen fuel, thereby improving its competitiveness in comparison to fossil fuels. In terms of the environment, it facilitates the shift towards more sustainable energy sources by decreasing the release of greenhouse gases.
This technique has practical applications in multiple industries, such as transportation, electricity generation, and chemical manufacture. Biaxial strain engineering can improve efficiency and reduce costs associated with hydrogen generation, thereby promoting hydrogen’s use as a sustainable energy carrier. Consequently, this can diminish the dependence on fossil fuels and contribute to a more sustainable energy future.
Comparative analysis to alternative methods:
Biaxial strain engineering provides significant benefits compared to conventional approaches, including increased efficiency and improved utilization of materials. Conventional approaches to enhancing HER performance typically rely on utilizing costly and scarce materials, such as platinum. Despite their efficiency, these approaches lack long-term viability and expansion potential. Conversely, biaxial strain engineering utilizes current materials and fabrication methods, resulting in a more economical and expandable solution. Chemical state and electronic structure analysis.
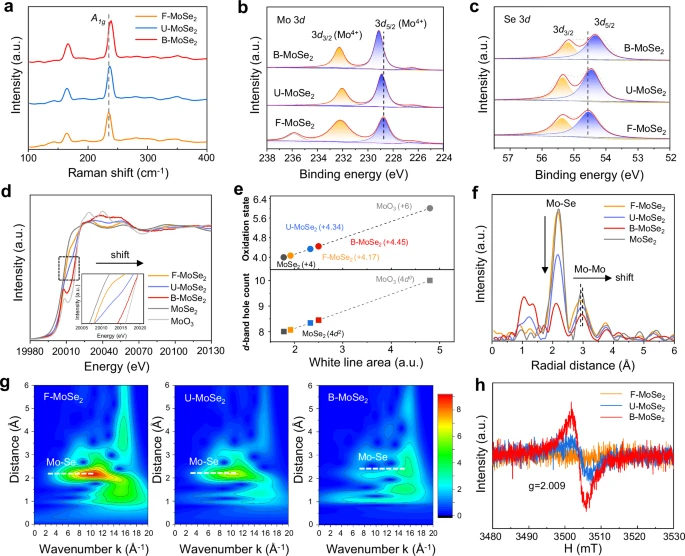
Examining specific instances or examples:
Real-world scenarios demonstrate the potential of biaxial strain-induced catalysts. Notable achievements include successful pilot projects in hydrogen production facilities that have significantly improved efficiency. We conducted a pilot project using strained NiFe catalysts in an alkaline electrolyzer. This project demonstrated a notable decrease in overpotential and a simultaneous increase in hydrogen production rates. These case studies offer significant insights for future implementations and showcase the tangible advantages of this method.
In conclusion:
One way to possibly speed up the alkaline hydrogen evolution reaction (HER) is to change the OH content using a biaxial strain. This strategy provides a means to achieve more efficient hydrogen production by improving the activity and stability of catalysts. Ongoing research and innovation are crucial to overcome existing limitations and fully exploit the potential of this technology.
Biaxial strain engineering further amplifies its economic and environmental advantages when incorporated into renewable energy sources like solar and wind. As the global shift towards cleaner energy sources takes place, the utilization of technologies such as biaxial strain-induced OH engineering will be essential in facilitating sustainable hydrogen production and mitigating greenhouse gas emissions.
Frequently Asked Questions:
1). What role does OH play in the hydrogen evolution reaction (HER) process?
Hydroxyl (OH) groups act as intermediates in the proton transfer mechanism during the hydrogen evolution reaction (HER), aiding in the conversion of water into hydrogen. In the alkaline hydrogen evolution reaction (HER), the catalyst surface must have hydroxyl (OH) groups to help move protons around and make the reaction go more quickly overall.
2). What is the mechanism by which biaxial strain enhances the performance of the hydrogen evolution reaction (HER)?
The electrical properties of catalysts are changed by biaxial strain. This makes them better at absorbing and stabilizing hydrogen intermediates, which speeds up the reaction. Strained catalysts can decrease the energy barriers for the hydrogen evolution process (HER) and enhance the reaction’s pace by altering the atomic lattice and electronic structure.
3). Which materials are best suited for biaxial strain-induced OH engineering?
Transition metal oxides, nitrides, and carbides, such as NiFe, CoP, and MoS2, are highly efficient materials for this function. These materials have the required structural characteristics to endure stress and demonstrate improved catalytic efficiency.
4). What are the primary obstacles in this domain?
The technical hurdles encompass creating a consistent distribution of strain, ensuring material stability throughout operational circumstances, and scaling up the production process. Ensuring material stability is of utmost importance, as catalysts that are under strain may experience alterations in their structure or deterioration as time passes.
5). What are the future possibilities for the alkaline hydrogen evolution reaction (HER)?
Potential future developments encompass advancements in catalyst design, incorporation with renewable energy systems, and wider utilization in diverse electrochemical processes. Ongoing research and development in strain engineering will accelerate the advancement of more efficient and stable strained catalysts, thereby amplifying their influence on sustainable hydrogen production.
For more chemistry blogs, visit chemistry Master