Table of Contents
Overview of Nickel(II)/BINOL-Catalyzed:
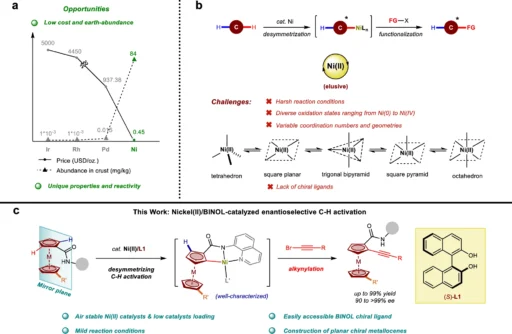
In the realm of organic chemistry, the ongoing struggle lies in discovering effective techniques to produce intricate structures with exceptional selectivity. Out of the different methods available, C-H activation is particularly notable for its effectiveness in directly modifying C-H bonds, which are usually considered unreactive. Researchers have found Nickel (II) to be a promising catalyst when combined with BINOL (1,1′-bi-2-naphthol) to achieve enantioselective C-H activation. This article talks about the complicated process of nickel(II)/BINOL-catalyzed enantioselective C-H activation. It focuses on how desymmetrization and kinetic resolution help make these reactions more efficient and selective.
Comprehending C-H Activation:
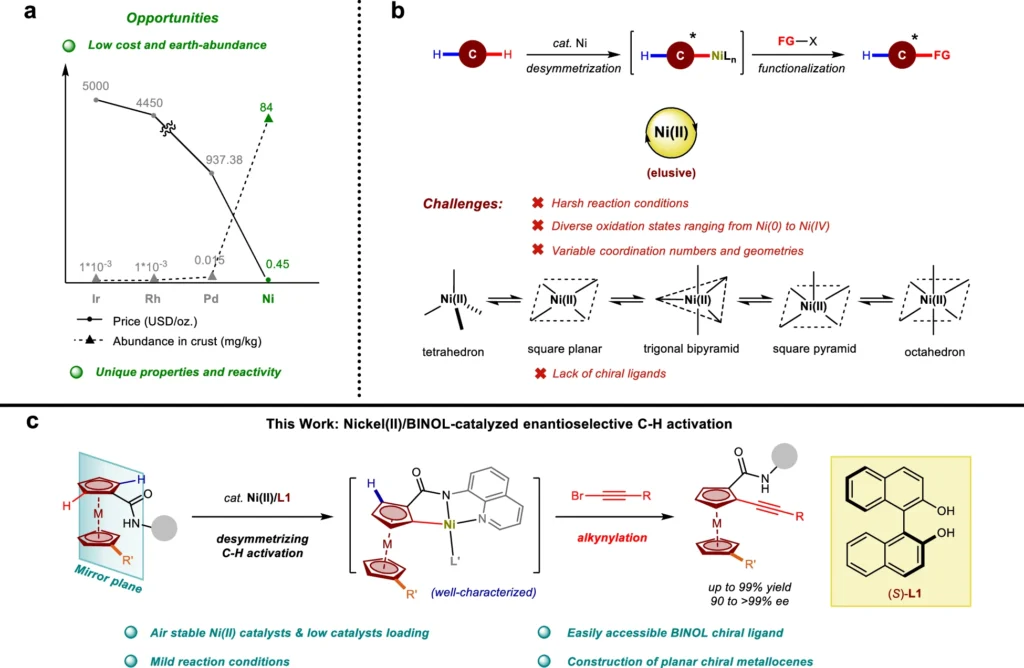
It is possible to change C-H bonds directly in organic compounds, so you don’t need substrates that have already been functionalized. This transition is particularly advantageous in organic synthesis since it enables chemists to change molecules more straightforwardly and efficiently while minimizing the use of atoms. Nevertheless, it is very hard to achieve selectivity in C-H activation because C-H bonds are so common and similar in organic molecules. A very important area of research is making catalytic systems that can get around the problems with old methods, which sometimes needed harsh conditions or didn’t have the accuracy needed for enantioselective synthesis. Nickel catalyzed enantioselective C-H activation.
Utilizing nickel (II) as a catalyst:
Nickel (II) is a popular catalyst for C-H activation processes because it has unique electrical properties and can keep different oxidation states stable. Contrary to conventional catalysts such as palladium or rhodium, nickel (II) provides a favorable combination of reactivity and cost-efficiency. It works well for coupling reactions because it can allow for both oxidative to its high catalytic efficiency and ability to operate under mild circumstances, C-H activation prefers Nickel(II). This is essential for preserving the integrity of delicate functional groups.
BINOL plays an important role in enantioselective catalysis:
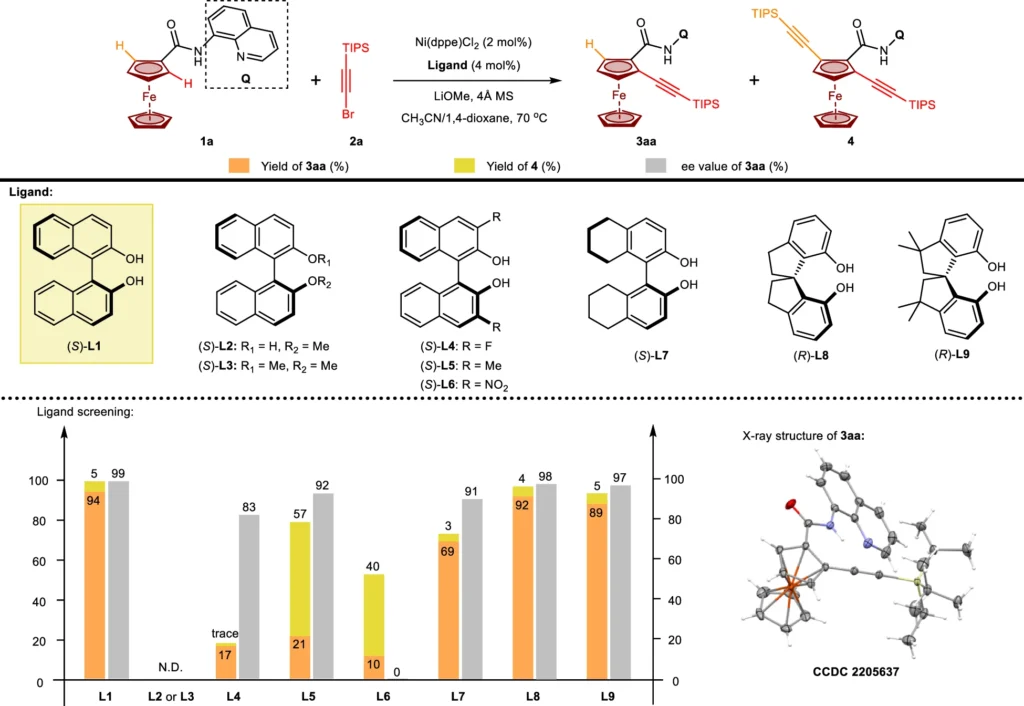
Because of its ability to produce significant levels of enantioselectivity, asymmetric catalysis has widely employed Binol, also known as 1,1′-bi-2-naphthol, a chiral ligand. The flat, rigid shape of BINOL and its ability to firmly attach to metal centers make it an ideal ligand for chiral catalysts. BINOL’s role in nickel(II)-catalyzed C-H activation is critical for determining the specific stereochemical result of the reaction. By creating a chiral environment around the nickel (II) center, BINOL makes sure that the C-H activation happens with a high level of enantioselectivity. This is critical for producing optically pure molecules. The optimization of chiral ligands for Ni(II)-catalyzed enantioselective C-H alkynylation.
Mechanism of Nickel(II)/BINOL-Catalyzed C–H Activation
The Nickel(II)/BINOL-catalyzed C-H activation operates through a series of essential stages that work together to achieve enantioselective functionalization. The coordination of the substrate to the Nickel(II)/BINOL-catalyzed center typically initiates the process, followed by the activation of a specific C-H bond. BINOL’s chiral environment frequently enhances the activation, ensuring a selective preference for one enantiomer over the other in the reaction. Following this, the next steps consist of introducing a functional group into the activated C-H bond and restoring the active catalyst, enabling the cycle to proceed.
Desymmetrization is a crucial factor in this process, as it selectively activates one of the two identical C-H bonds in a symmetrical molecule. The process of selective activation not only incorporates chirality into the molecule but also improves the reaction’s overall enantioselectivity. Kinetic resolution improves selectivity by specifically reacting one enantiomer of a racemic mixture, resulting in a highly enantioenriched product. Plausible mechanism.
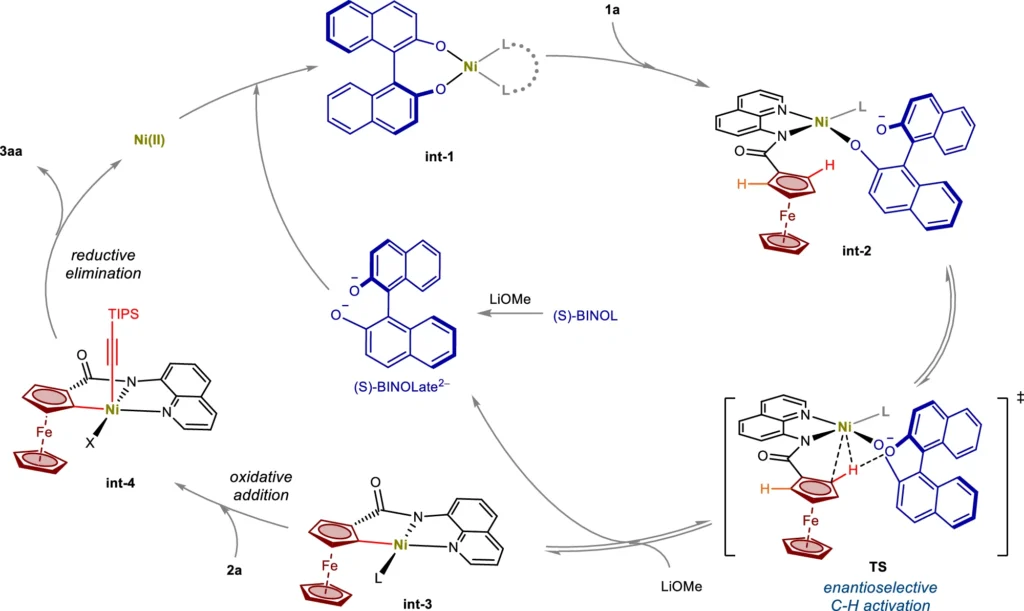
Organic synthesis uses a process known as desymmetrization:
Desymmetrization is a highly effective approach in organic synthesis, especially when considering C-H activation. Chemists can generate intricate, chiral substances from basic, achiral starting materials by deliberately disrupting the symmetry of a molecule. When nickel(II) and binol are used to speed up a reaction, desymmetrization usually means activating a single C-H bond in a molecule that has many identical C-H bonds. The process of selectively activating specific molecules is essential to achieve a high degree of enantioselectivity. This step is frequently the determining factor in the reaction’s overall success.
Enantioselective synthesis uses the kinetic resolution technique:
Kinetic resolution is a crucial technique in enantioselective synthesis, especially for handling racemic mixtures. In a normal kinetic resolution, the catalyst reacts more with one enantiomer than the other. This separates the two enantiomers and makes it possible to separate the desired chiral product with a high enantiomeric excess. There is a very good way to make molecules with high optical purity, even from starting materials that are racemic or achiral, using Nickel(II)/BINOL to help with desymmetrization and kinetic resolution in C-H activation. Synthetic transformations of planar chiral ferrocenes.
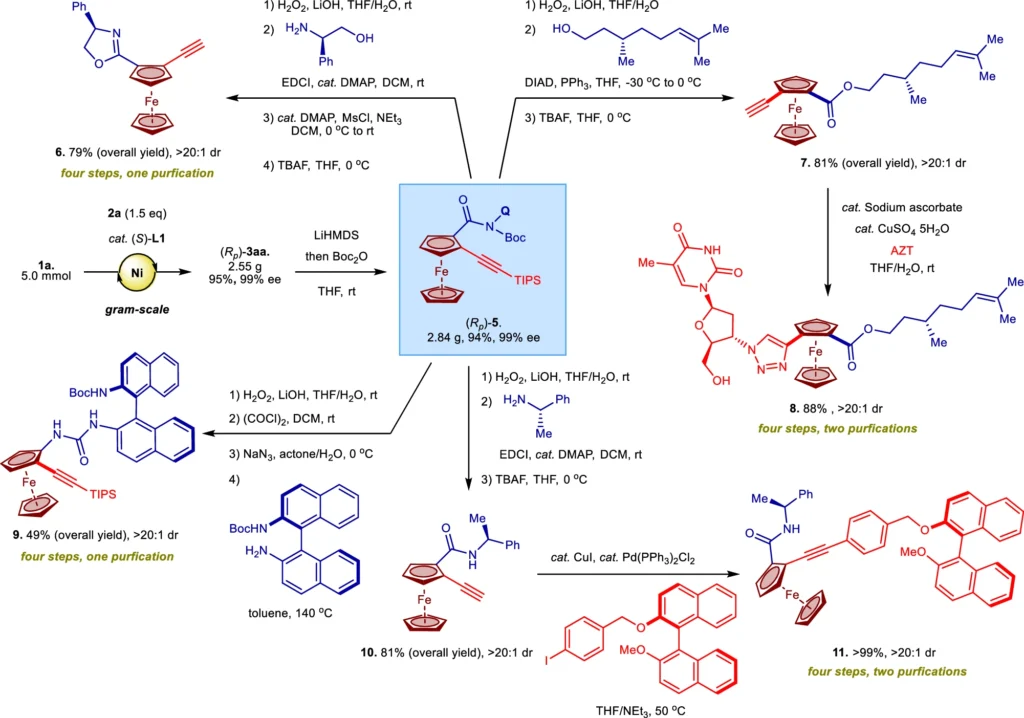
Uses of Nickel (II)/BINOL-Catalyzed Reactions:
Nickel(II)/BINOL-catalyzed enantioselective C-H activation has numerous and varied uses. An area of great importance is the utilization of this technique in the creation of intricate natural compounds and medicinal drugs Adding chirality to a molecule through C-H activation makes it easier to make stereochemically complex structures, which is common in pharmaceutical compounds. y seen in medicinal compounds. Moreover, the production of innovative materials and polymers, where precise manipulation of molecular structure is essential in establishing the characteristics of the result, has utilized these catalysts.
Benefits of Utilizing Nickel(II)/BINOL-Catalyzed:
The use of Nickel(II)/BINOL-catalyzed for enantioselective C-H activation has numerous advantages. The primary advantage is the ability to achieve a high level of enantioselectivity, which is essential for synthesizing optically active molecules. Also, these catalysts often work in mild reaction conditions, which protects the integrity of delicate functional groups and keeps the reaction’s impact on the environment to a minimum. In addition, nickel (II) is a plentiful and affordable metal, making these catalytic systems more economically efficient than those relying on precious metals such as palladium or rhodium. Illustrating the enantio-determining step.
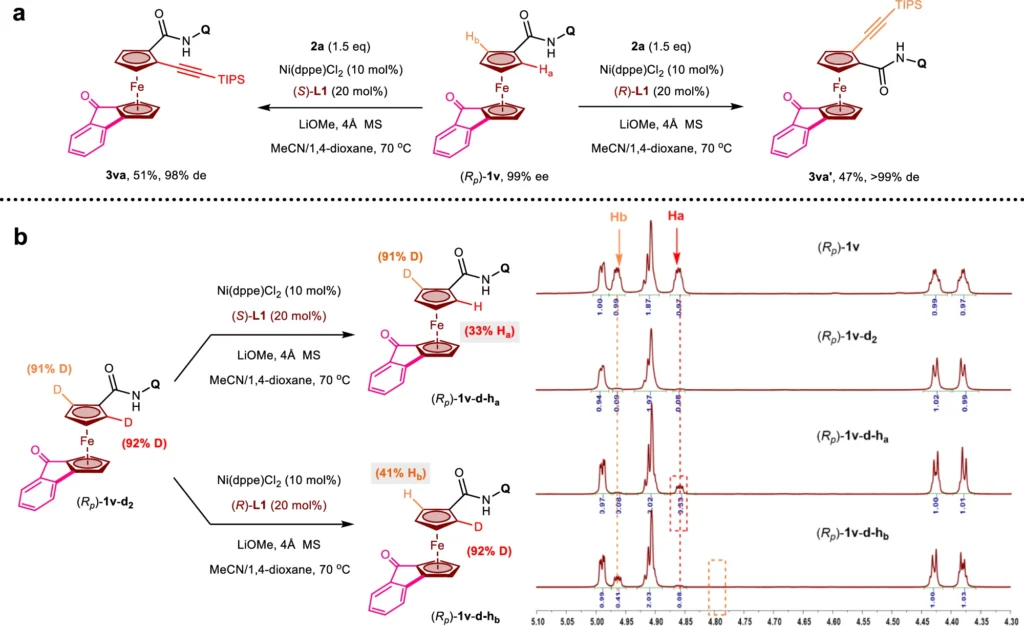
Obstacles and Constraints:
Despite the numerous benefits, nickel(II)/BINOL-catalyzed C-H activation is not without problems and restrictions. The restricted range of substrates that this approach can efficiently activate is a major obstacle. Although Nickel(II)/BINOL-catalyzed exhibits good selectivity, they may not be appropriate for all C-H bonds, especially those that are extremely electron-rich or sterically hindered. Moreover, these catalysts may have a restricted functional group tolerance, necessitating careful deliberation while selecting substrates and reaction conditions. Environmental and safety concerns can also arise, particularly when using large quantities of BINOL or handling potentially dangerous nickel (II) salts.
Recent Advances and Innovations:
Recent developments in Nickel(II)/BINOL-catalyzed have primarily aimed at broadening the range of substrates and enhancing the efficiency of the catalytic process. Scientists have formulated novel derivatives of BINOL and other chiral ligands to enhance the selectivity and reactivity of these systems. In addition, scientists have investigated the utilization of alternate reaction solvents and parameters to minimize the ecological footprint and enhance the scalability of these reactions. The advancements have created fresh opportunities for utilizing Nickel(II)/BINOL-catalyzed in the production of intricate compounds and substances.
The catalytic system undergoes a comparative analysis with other systems:
Nickel (II)/BINOL catalysts provide some clear advantages when compared to other catalytic systems, especially in terms of cost and selectivity. Although palladium and rhodium catalysts exhibit higher reactivity, they are also more costly and may have lower selectivity, especially in enantioselective reactions. On the other hand, nickel (II)/BINOL systems present a more equitable strategy, delivering excellent selectivity at a reduced expense, rendering them a compelling choice for both academic and industrial chemists. Case studies in enantioselective synthesis have shown how useful these catalysts are in some different situations, which makes them even more valuable in the field of organic synthesis. Kinetic resolution.
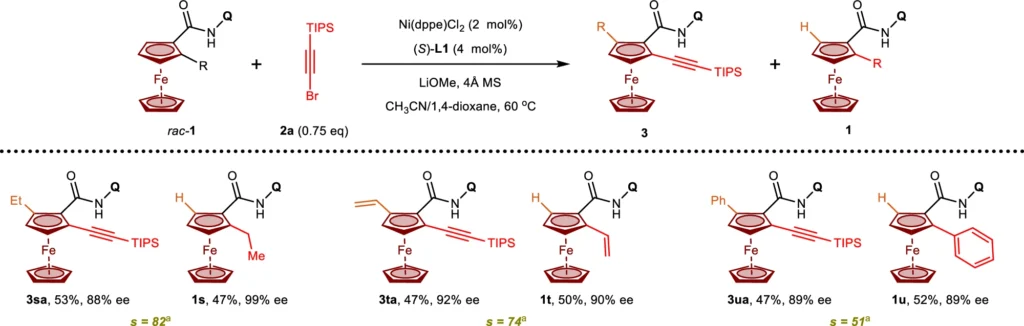
Methodology and Experimental Settings:
For the standard reaction conditions for Nickel(II)/BINOL-catalyzed C-H activation, you need to use a Nickel(II) precursor, BINOL as a chiral ligand, and the right base or oxidant. The choice of solvent and temperature can have a significant impact on the reaction’s result, with numerous procedures suggesting lower temperatures and non-polar solvents to achieve the best selectivity. It is crucial to meticulously regulate the stoichiometry of the reagents to prevent excessive reactions or the formation of undesired byproducts. Instances from the literature frequently emphasize the importance of maximizing these circumstances to achieve consistent and productive outcomes.
In conclusion:
In contemporary chemical synthesis, the use of nickel(II)/BINOL-catalyzed enantioselective C-H activation is a highly effective and adaptable method. Through desymmetrization and kinetic resolution, this catalytic system makes it possible to make complex, chiral compounds quickly and selectively. Nickel(II)/binol catalysts are useful tools for chemists because they are cost-effective, selective, and don’t harm the environment. However, they do come with some problems and restrictions. With the continuous progress of research, we may anticipate an influx of even more groundbreaking applications and advancements in this captivating sector.
FAQs:
1). What are the advantageous properties of BINOL as a ligand for enantioselective catalysis?
Due to its rigid and chiral structure and good coordination of metal centers, BINOL is a good material for creating high levels of enantioselectivity in catalytic processes.
2). What role does desymmetrization play in enhancing enantioselectivity?
Desymmetrization is a process that specifically activates one of the identical C-H bonds in a symmetrical molecule. This introduces chirality and enhances the reaction’s enantioselectivity.
3). Compared to other transition metals, the use of nickel (II) offers certain benefits.
Nickel (II) has a favorable equilibrium between reactivity and cost, functions effectively in moderate settings, and is more economical compared to valuable metals such as palladium or rhodium.
4). Are nickel (II) and nickel (BINOL) catalysts suitable for use in large-scale industrial applications?
Nickel (II)/BINOL catalysts are useful for scaling up in industrial processes because of their cost-effectiveness, selectivity, and comparatively mild reaction conditions.
5). What are the potential avenues for further investigation into C-H activation?
Future research could prioritize the expansion of the range of substrates, enhancing the efficiency of catalysts, and devising environmentally friendly and sustainable reaction conditions for C-H activation.
For more chemistry blogs, visit chemistry Master