Table of Contents
Preface of Non-Classical Boron- Cluster:
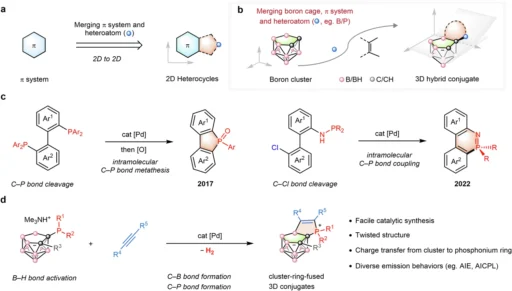
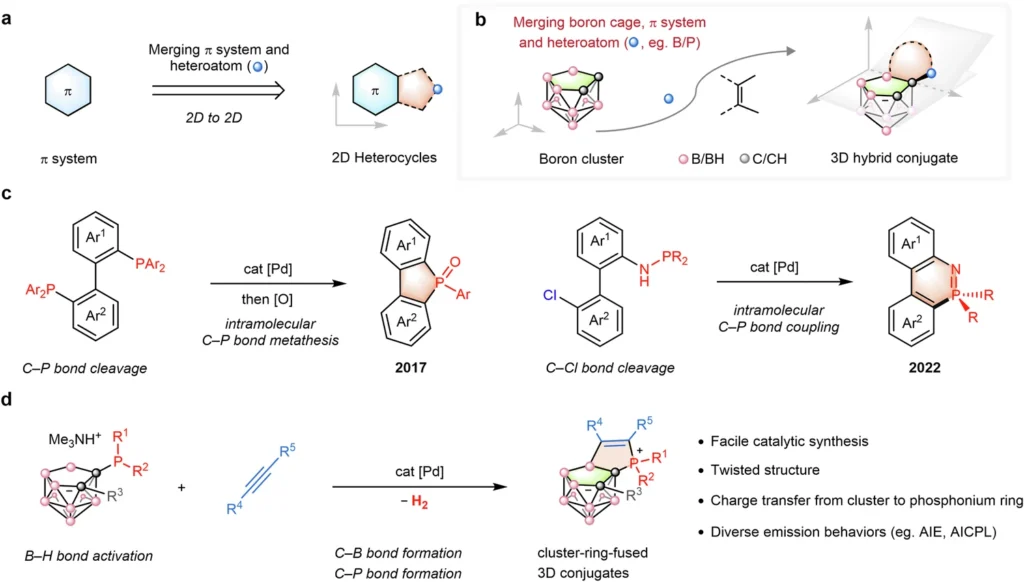
In contemporary chemistry, designing and synthesizing new compounds with distinctive features are critical to improving technology and scientific knowledge. Non-classical Boron-Cluster, recognized for their atypical bond configurations and distinctive geometric forms, have consistently intrigued researchers. These clusters display electrical, photonic, and reactivity properties, rendering them exceptionally advantageous across several applications. The conjugation of boron-clusters with phosphonium ions yields conjugates that exhibit considerable potential in medicine, catalysis, material science, and other fields.
This article will talk about couple-close fabrication of non-classical boron-cluster-phosphonium conjugates, a new way to make molecules that makes it easier to make them correctly. We will investigate the characteristics of boron clusters and phosphonium ions, analyze their conjugation, and assess the significance of this chemistry in practical applications. The couple-close methodology effectively integrates these two disparate chemical entities, yielding molecules with remarkable stability, reactivity, and promise. Background and development.
Boron-Clusters: An Examination of the Fundamentals
Comprehending Boron in Chemistry:
Boron, located in Group 13, is a distinctive element in the periodic table. It possesses characteristics that distinguish it from numerous other components. In contrast to carbon, which establishes robust two-center bonds in organic compounds, boron can create multicenter connections wherein many atoms share a pair of electrons. This results in boron clusters—cage-like formations maintained by these atypical bonds.
Boron’s diminutive size and the versatility of its bonding enable it to assume multiple structural configurations, such as planar, polyhedral, and cage-like forms. The unique bonding characteristics of boron significantly contribute to its importance in the development of new materials, especially when researchers seek to utilize its electrical or optical capabilities.
Boron-Clusters: Classical vs. Non-Classical
We typically categorize boron-clusters into two types: classical and non-classical. Classical boron-clusters have clear and predictable bonding patterns. They usually have clos-, nido-, or arachno-type structures. These structures follow the Wade-Mingos rules, which make it easier to guess the shapes and connections of boron hydrides and similar groups.
Non-classical boron-clusters, however, diverge from these anticipated patterns. They demonstrate distinctive bonding configurations that contest traditional comprehension. These non-classical clusters frequently exhibit multicenter bonding, wherein several atoms contribute electrons to a link that transcends a mere pair of atoms. This leads to more intricate electronic structures and characteristics, rendering non-classical boron clusters especially appealing for advanced applications.
Non-Classical Boron-Clusters’ Structure, Bonding, and Characteristics
The architecture of non-classical boron clusters is generally cage-like, with multiple boron atoms constituting the vertices of a polyhedral formation. In several instances, these clusters encompass supplementary atoms, such as hydrogen, which reinforce the structure’s stability. In these clusters, the bonding is extensively delocalized, with electrons distributed among several boron atoms, resulting in attributes such as elevated electron density, distinctive magnetic properties, and increased reactivity.
The electronic plasticity of non-classical boron-clusters is one of their most notable characteristics. These clusters are useful in catalysis (where electron transfer is important) and materials research (where electronic properties are key to functionality) because their electrons are spread out and can take part in many electronic transitions.
The Significance of Non-Classical Clusters in Contemporary Chemistry:
Non-classical boron-clusters have properties that make them useful in many situations, especially where high thermal stability, unique electrical properties, and the ability to form strong, directed interactions with other chemical species are needed. Materials for catalysts, sensing, and drug delivery systems increasingly use these clusters. Moreover, their electrical characteristics render them significant for the advancement of novel semiconductor materials and nanotechnology applications.
Phosphonium Ions and Their Significance in Chemistry:
Overview of Phosphonium Ions:
Phosphonium ions are cationic entities comprising a phosphorus atom covalently linked to four substituents. Numerous synthetic processes extensively utilize them as an essential element in organophosphorus chemistry. Salts predominantly contain phosphonium ions, where the phosphorus atom bears a formal positive charge. The positive charge renders phosphonium ions very reactive, particularly in nucleophilic processes.
Phosphonium Ions’ Chemical Characteristics and Reactivity:
Four organic groups, ranging from basic alkyl groups to more intricate aromatic or heteroaromatic groups, generally encircle the central phosphorus atom in a phosphonium ion. Phosphonium ions are stable in many situations because of their tetrahedral shape, but they are very reactive when they come into contact with electron-rich species (nucleophiles) because of their positive charge.
Phosphonium ions are important in organic synthesis because they can function as intermediates in a variety of processes. The Wittig reaction frequently uses them to generate ylides, which are entities capable of facilitating the formation of double bonds between carbon atoms. Because they can make reactive intermediates, phosphonium ions are perfect for joining with other reactive substances, such as boron-clusters.
The Importance of Phosphonium Ions in Organophosphorus Chemistry:
Organophosphorus chemistry has thoroughly investigated phosphonium ions, where they serve as reagents in numerous synthetic transformations. Because they can stabilize negative charges and make stable ylides, they are needed to make new bonds between carbon atoms and between heteroatoms and carbon atoms.
Organic synthesis, materials science, and catalysis utilize phosphonium ions. Their ability to interact with various functional groups makes it easier to create complex molecular structures, such as those that contain boron clusters. The adaptability of phosphonium ions has made them an important tool for researchers looking to create novel compounds with improved properties.
Reasons Phosphonium Ions Are Optimal for Conjugation with Boron-Clusters:
The positive charge of phosphonium ions and their ability to form stable covalent bonds with other substances make them perfect for conjugation with boron clusters. Phosphonium ions, when coupled to boron clusters, can augment the overall stability and reactivity of the resultant molecule. Also, the phosphonium ion’s electrical properties can be improved by adding them to the boron clusters. This creates synergistic effects that make the conjugate more useful. Reaction discovery and mechanistic studies.
Conjugation in Chemistry: An Essential Principle
What is conjugation in chemistry?
In chemistry, conjugation denotes the process of combining two separate chemical entities to create a novel molecule. It is common for covalent bonds to form between the two parts during this process. However, non-covalent interactions like electrostatic forces and hydrogen bonding may also play a role. The final conjugate often has properties that combine those of the two original parts. This makes conjugation an important method for making compounds with specific functions.
The article discusses the categories of conjugates and their functions in contemporary science.
Various scientific fields, including pharmaceuticals and materials research, utilize conjugates. In medicine, we employ conjugates such as antibody-drug conjugates (ADCs) to specifically target cells or tissues, thereby facilitating the direct delivery of medications to their site of action. In materials research, conjugates are employed to develop novel materials with improved attributes, such as augmented strength, conductivity, or reactivity.
When boron cluster-phosphonium conjugates are joined together, they form a molecule that combines the unique properties of both the boron-cluster and the phosphonium ion. These conjugates are particularly advantageous for applications necessitating both stability and reactivity, such as catalysis or materials research.
Conjugation of Boron-Clusters with Phosphonium Ions:
The conjugation of boron-clusters with phosphonium ions often entails the establishment of a covalent link between the two entities. Typically, a couple-close reaction establishes this bond, using a catalyst to speed up the union of the two components. The conjugate that was made is more stable and reactive, and it has unique electrical properties because the boron cluster and the phosphonium ion joined together.
A linker molecule often connects the phosphonium ion to the boron-cluster, stabilizing the conjugate and enhancing its overall activity. The selection of a linker can profoundly influence the characteristics of the conjugate, prompting researchers to incessantly investigate novel methods to enhance this facet of the conjugation process.
Covalent and non-covalent interactions are present in Boron-Phosphonium conjugates:
Covalent bonds are generally the predominant mode of interaction between boron clusters and phosphonium conjugates. These bonds are robust and durable, guaranteeing that the conjugate remains unaltered under diverse situations. Even so, non-covalent interactions such as electrostatic forces and hydrogen bonds may help keep the conjugate stable or make it more reactive.
The phosphonium ion’s positive charge can electrostatically interact with the electron-dense areas of the boron-cluster, resulting in improved conjugate stability. When the conjugate encounters extreme conditions, like in catalysis or materials research, non-covalent interactions become particularly significant.
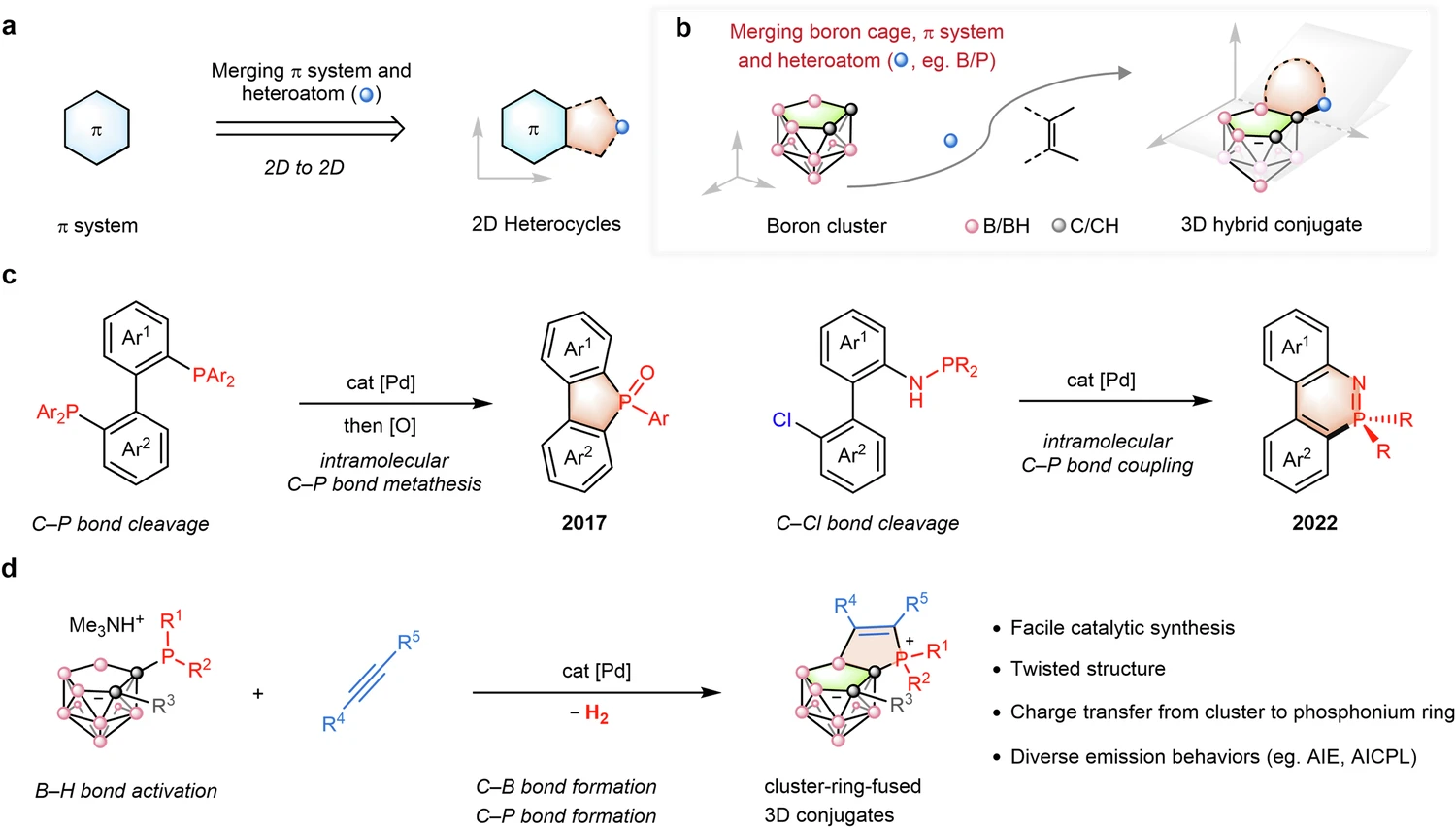
Couple-Close Construction: A Revolutionary Synthesis
What is couple-close construction in chemistry?
Couple-close construction is a contemporary synthetic method that merges two separate molecular entities into a single, stable product. This method is especially advantageous for synthesizing conjugates, as it facilitates meticulous regulation of the bonding procedure. When it comes to boron-cluster-phosphonium conjugates, couple-close building makes it easier for strong, stable bonds to form between the boron-cluster and the phosphonium ion.
The fundamentals of couple-close techniques:
The fundamental premise of couple-close construction involves employing a catalyst to promote the union of two molecular entities. The catalyst helps to turn on both the boron-cluster and the phosphonium ion, which lets them react and form a covalent bond. This technique is exceptionally efficient, facilitating the controlled synthesis of intricate molecular architectures.
The couple-close approach frequently employs transition metal catalysts, including palladium or platinum. These metals are very good at helping different chemicals form covalent bonds with each other, which makes them useful for use in close-coupling reactions.
Mechanism of Couple-Close Construction in Boron Cluster-Phosphonium Synthesis:
Usually, the process of making boron cluster-phosphonium conjugates starts with turning on the boron cluster during the couple-close building phase. You can accomplish the activation by using a catalyst that exposes the reactive sites on the boron cluster. When the boron cluster is activated, it can react with the phosphonium ion, forming a covalent connection between the two entities.
The use of a catalyst in this process is critical because it facilitates the reaction’s effective and selective progression. In the absence of a catalyst, the boron cluster and the phosphonium ion may either fail to react or produce undesirable byproducts. Using a catalyst allows researchers to regulate the reaction and ensure that the desired compound is formed.
The Benefits of Couple-Close Techniques:
The primary advantage of the couple-close technique is its capacity to generate intricate molecular architectures in a regulated and efficient manner. Making boron cluster-phosphonium conjugates is very important because the compounds that are made often have complicated structures that need careful control over the bonding process.
Furthermore, the couple-close technique facilitates the formation of stable conjugates capable of enduring diverse environments. This is essential for applications where the conjugate may encounter severe conditions, such as in catalysis or materials science. The modification of functional molecules.
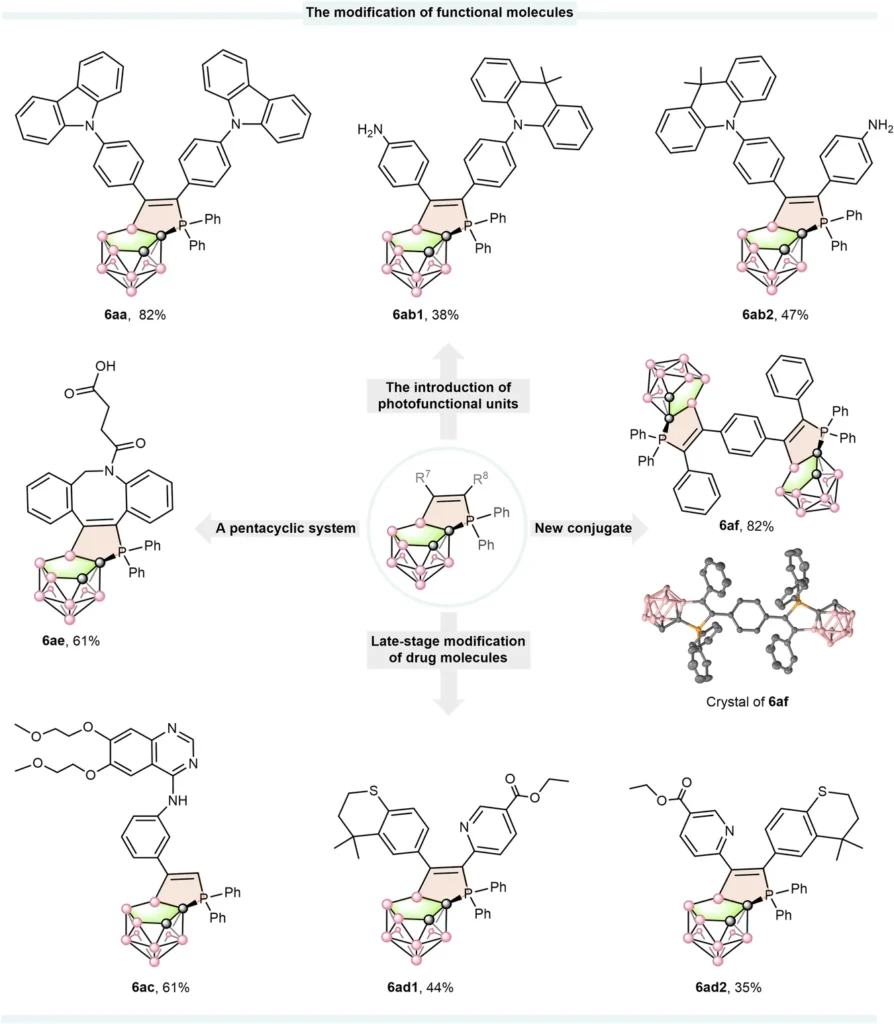
Synthesis Methods for Boron Cluster-Phosphonium Conjugates:
An Overview of Organoboron Chemistry:
Organoboron chemistry is a discipline that focuses on the study and application of compounds with boron-carbon bonds. This domain has garnered considerable interest in recent years owing to the distinctive characteristics of boron-containing compounds, especially within the realms of materials science, catalysis, and medicine.
Organoboron chemistry plays a critical role in the production of boron cluster-phosphonium conjugates. The synthesis of boron clusters typically necessitates specialized procedures and reagents specific to organoboron chemistry. Researchers can synthesize boron clusters with defined shapes and properties using these procedures, and then couple them with phosphonium ions.
Essential Reagents and Catalysts for Boron Cluster Synthesis:
Boron cluster synthesis generally requires specialized reagents and catalysts to promote the formation of boron-boron and boron-carbon bonds. Typical reagents employed in the synthesis of boron clusters comprise boron hydrides (e.g., B_2H_6) and boron halides (e.g., BCl_3). These reagents supply the requisite boron atoms for cluster formation.
Together with these reagents, catalysts are essential in the synthesis of boron clusters. We frequently employ transition metal catalysts such as palladium, platinum, and rhodium to promote the formation of boron-boron bonds. These catalysts facilitate the activation of boron atoms, enabling them to react and construct the ideal cluster structure.
General Synthetic Routes for Boron Cluster-Phosphonium Conjugates:
The production of boron cluster-phosphonium conjugates often entails a multi-step procedure. Organoboron chemistry techniques produce the boron cluster. A catalyst activates the boron cluster upon its formation, preparing it for conjugation with the phosphonium ion.
The reaction mixture then receives the phosphonium ion, which interacts with the activated boron cluster to form a covalent bond. A transition metal catalyst frequently facilitates this process, ensuring the reaction proceeds rapidly and selectively.
Ultimately, we separate and purify the boron cluster-phosphonium conjugate. This process is essential, as it guarantees that the final product is devoid of contaminants and undesirable byproducts. Applications for the purified conjugate range from catalysis to materials research.
Transition Metals’ Role in Boron-Phosphonium Conjugation:
Transition metals are pivotal in the conjugation of boron clusters and phosphonium ions. Palladium and platinum are extremely proficient at promoting the development of covalent connections between various chemical species. Transition metal catalysts help both the boron cluster and the phosphonium ion get activated in boron cluster-phosphonium conjugates. This lets them react and make a stable conjugate.
Transition metals, in addition to their role in bond formation, can also regulate the selectivity of the reaction. This is especially crucial in the synthesis of complex conjugates, where several reactive sites may exist. Utilizing a transition metal catalyst enables researchers to guarantee the creation of the desired bond while preventing the generation of undesirable byproducts.
Challenges and Complexities in Couple-Close Synthesis:
Understanding the Difficulties in Conjugating Boron Clusters and Phosphonium Ions: Comprehending the Challenges in Conjugating Boron Clusters and Phosphonium Ions
Although the couple-close construction style is highly effective, it has certain drawbacks. One of the principal challenges in conjugating boron clusters and phosphonium ions is establishing a stable link between the two entities. Boron clusters, especially non-classical varieties, may exhibit significant reactivity or instability under specific conditions. This complicates the formation of a stable conjugate without specialized procedures and catalysts.
The boron cluster’s dimensions and configuration can cause steric hindrance, impeding the phosphonium ion’s ability to approach and react with the cluster. This is particularly true for larger boron clusters, where the presence of several boron atoms may obstruct the phosphonium ion’s approach.
Mitigating steric hindrance and bonding complications:
Researchers have devised numerous techniques to address these difficulties. One method involves utilizing smaller boron clusters, which exhibit fewer steric hindrances and facilitate easier conjugation with phosphonium ions. Alternatively, researchers may employ linker molecules to establish a separation between the boron cluster and the phosphonium ion, facilitating bond formation.
An alternative method involves employing protective groups to temporarily obstruct reactive sites on the boron cluster during the conjugation process. We can eliminate the protective group’s post-conjugate formation, resulting in a stable boron cluster-phosphonium conjugate.
Stability Concerns in Boron Cluster-Phosphonium Conjugates:
When the boron cluster-phosphonium conjugate forms, stability becomes a critical issue. Non-classical boron clusters have significant reactivity, rendering them susceptible to breakdown under specific conditions. This is particularly relevant in environments where the conjugate encounters heat, light, or reactive substances.
Researchers are investigating various techniques to resolve these stability difficulties. One strategy involves altering the configuration of the boron cluster to enhance its stability. You can achieve this by adding supplementary atoms or functional groups that aid in the cluster’s stabilization. An alternative method involves employing stabilizing agents, such as ligands or solvents, to safeguard the conjugate from degradation.
Strategies to Mitigate These Challenges:
Researchers have devised numerous ways to guarantee the successful synthesis of boron cluster-phosphonium conjugates. One method involves employing sophisticated catalytic devices that enhance the conjugation process while reducing the generation of undesirable byproducts. Transition metals are often used in these catalytic systems to control how the boron cluster and the phosphonium ion react.
Another strategy involves optimizing the reaction parameters, including temperature, pressure, and solvent selection, to establish an environment favorable for the production of stable conjugates. By meticulously regulating these variables, researchers can enhance the efficacy of the conjugation process and augment the overall stability of the resultant conjugate. Structural investigations.
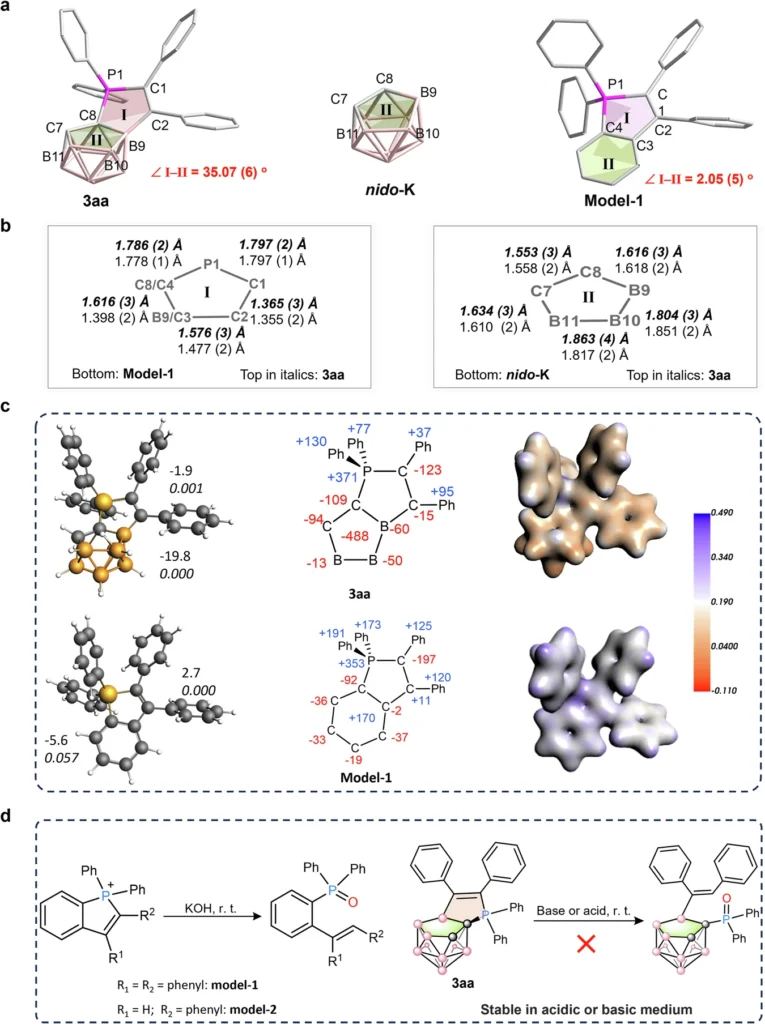
Recent Advances in Non-Classical Boron Cluster Chemistry:
Recent Advancements in Non-Classical Boron Cluster Chemistry:
Recent years have witnessed substantial progress in non-classical boron cluster chemistry. Investigators have established novel synthetic methodologies to facilitate the regulated production of these distinctive structures, and are examining strategies to exploit their atypical bonding configurations for diverse applications.
A significant advancement in this domain is the identification of novel non-classical boron clusters that demonstrate unparalleled stability and reactivity. Sophisticated catalytic systems and tailored reaction conditions have produced these clusters, enabling researchers to produce boron clusters with distinct electrical and optical characteristics.
Innovative Approaches to Boron Cluster Synthesis and Stabilization:
Researchers have devised several innovative techniques for the synthesis and stabilization of non-classical boron clusters. One method uses transition metal catalysts to promote the controlled production of boron-boron bonds. Researchers can synthesize boron clusters with defined shapes and properties using these catalysts, which can subsequently find numerous applications.
An alternative method employs stabilizing substances, including ligands or solvents, to safeguard the boron cluster from deterioration. These stabilizing chemicals preserve the integrity of the boron cluster, enabling its application in diverse hostile settings.
The Function of Advanced Catalysis in Non-Classical Boron Cluster Chemistry:
Catalysis is pivotal in the synthesis of non-classical boron clusters. Engineers have engineered advanced catalytic systems, particularly those utilizing transition metals, to enable the controlled production of boron-boron bonds. These catalysts facilitate the activation of boron atoms, enabling them to react and generate the intended cluster structure.
Catalysts not only promote bond formation, but they also regulate the reaction’s selectivity. This is especially crucial in the synthesis of non-classical boron clusters, which may contain several reactive sites. Utilizing a catalyst, researchers can guarantee the production of the desired connection while preventing the generation of undesirable byproducts.
Utilization of These Advances in Phosphonium Conjugation:
The progress in non-classical boron cluster chemistry has considerable implications for the conjugation of these clusters with phosphonium ions. The capacity to synthesize boron clusters with defined structures and characteristics enables researchers to formulate conjugates customized for certain uses. Using complex catalyst systems makes it easier for boron clusters to join with phosphonium ions, creating stable and reactive compounds that can be used in many areas.
Utilization of Boron Cluster-Phosphonium Conjugates:
Medical Applications: Pharmaceutical Delivery, Imaging, and Diagnostic Procedures
The application of boron cluster-phosphonium conjugates in medicine is highly promising. These conjugates possess the potential for use in diverse medicinal applications, including medication delivery, imaging, and diagnostics.
Drug delivery can utilize boron cluster-phosphonium conjugates to selectively target specific cells or tissues within the body. Boron clusters have unique properties that make it easier to find them inside an organism, and the phosphonium ion can make the conjugate more stable and reactive. Boron cluster-phosphonium conjugates work best for targeted drug delivery systems because they allow drugs to be delivered directly to the site of action, which lowers side effects and increases effectiveness.
Boron cluster-phosphonium conjugates serve as contrast agents in imaging modalities, including MRI and PET scans. Because boron clusters have unique electrical properties, they can be used for imaging. The phosphonium ion can make the conjugate more stable and biocompatible.
Diagnostics can use boron cluster-phosphonium conjugates to pinpoint specific biomarkers or disease conditions. Customizing the conjugate’s reactivity to interact with specific molecules enables the development of highly sensitive diagnostic assays.
Applications of Catalysis and Materials Science:
Boron cluster-phosphonium conjugates possess considerable potential in catalysis and materials research. These conjugates’ distinctive electrical and reactivity properties make them excellent candidates for a variety of catalytic processes.
Boron cluster-phosphonium conjugates can speed up a wide range of chemical reactions, from simple bond formation to complex multi-step processes. You can adjust the reactivity of the boron cluster to enhance the efficiency of the reaction, and the phosphonium ion can stabilize the catalytic intermediate.
Materials science can utilize boron cluster-phosphonium conjugates to develop novel materials with improved characteristics. These materials are applicable in diverse fields, including electronics and energy storage. Because of the unique properties of boron clusters and the stability of phosphonium ions, these conjugates are perfect for making high-performance materials.
Prospects for Application in Electronic and Photonic Devices:
Because boron cluster-phosphonium conjugates have unique electrical properties, they are excellent choices for use in electronic and photonic devices. We can utilize these conjugates to develop novel semiconductors applicable in diverse fields, from solar cells to transistors.
Photonic devices can use boron cluster-phosphonium conjugates to fabricate materials that interact with light in innovative ways. These materials are applicable in light-emitting diodes (LEDs), lasers, and optical sensors. The ability to adjust the boron cluster’s electrical properties makes these conjugates exceptionally adaptable, allowing for the creation of materials with distinct optical attributes.
Additional Emerging Applications in Renewable Energy and Nanotechnology:
Boron cluster-phosphonium conjugates possess the potential for diverse applications, especially in renewable energy and nanotechnology. Renewable energy can utilize these conjugates to develop novel catalysts for energy conversion processes, such as hydrogen creation from water and carbon dioxide reduction to valuable fuels.
Nanotechnology can utilize boron cluster-phosphonium conjugates to synthesize novel nanomaterials with distinct characteristics. These nanoparticles are applicable in diverse fields, including medicine delivery and environmental sensing. These conjugates are perfect for nanotechnology because they can be used to change the size and shape of the boron cluster and the phosphonium ion will stay stable.
Mechanistic insights into coupled-closure reactions:
The Couple-Close Construction Reaction Mechanism:
In the couple-close building reaction, both the boron cluster and the phosphonium ion are usually activated. This is followed by the formation of a covalent bond between the two. A catalyst facilitates this process by reducing the reaction’s activation energy and ensuring the formation of the desired bond.
The reaction often occurs in a sequence of phases, commencing with the activation of the boron cluster. This activation could lead to the creation of reactive intermediates, such as boron radicals or cations, which are very reactive and easily bond with other things.
When the boron cluster is activated, it can interact with the phosphonium ion, forming a covalent connection between the two entities. A catalyst frequently aids in the development of this bond, stabilizing the reaction’s transition state and ensuring the production of the desired bond.
The Function of Intermediates in Boron-Phosphonium Conjugation:
Intermediates are essential in the couple-close building reaction, especially in the activation of the boron cluster. These intermediates are frequently highly reactive entities, such as boron radicals or boron cations, capable of readily forming bonds with other species.
The catalyst typically facilitates the production of these intermediates by reducing the reaction’s activation energy and stabilizing the intermediate species. After the intermediate is formed, it can react with the phosphonium ion to produce the required conjugate.
The production of intermediates may occasionally induce undesirable side reactions, leading to the generation of byproducts. Researchers carefully control the reaction conditions and use catalysts that are very selective for the bond formation they want to see. This keeps the production of unwanted byproducts to a minimum.
Coupled Reactions: Thermodynamic and Kinetic Considerations
Both thermodynamic and kinetic variables influence the couple-close building reaction. Based on thermodynamics, the reaction must be good because the boron cluster-phosphonium conjugate must have less energy than the reactants when it is formed.
The reaction must occur at a feasible rate, indicating that the activation energy must be sufficiently low for the reaction to transpire within a practical duration. The utilization of a catalyst is essential for reducing activation energy and facilitating the reaction at an acceptable rate.
Alongside the thermodynamic and kinetic variables, researchers also take into account the selectivity of the reaction. This is especially crucial in the synthesis of complex conjugates, where several reactive sites may exist. Using a selective catalyst enables researchers to guarantee the creation of the desired connection while preventing the generation of undesirable byproducts.
The Function of Ligands and Catalysts in Improving Efficiency:
Ligands and catalysts play an important role in the couple-close building reaction, increasing both the process’s efficiency and selectivity. Ligands frequently stabilize the transition state of a reaction, hence reducing the activation energy and facilitating the formation of the desired bond.
When transition metals are used as catalysts, they help the boron cluster and the phosphonium ion form covalent bonds. These helpers make it easier for both the boron cluster and the phosphonium ion to become active. This lets them react and form a stable conjugate.
Catalysts not only improve the reaction’s efficiency, but they also regulate its selectivity. This is especially crucial in the synthesis of complex conjugates, where several reactive sites may exist. Using a selective catalyst enables researchers to guarantee the creation of the desired connection while preventing the generation of undesirable byproducts. Photophysical properties of the selected compounds.
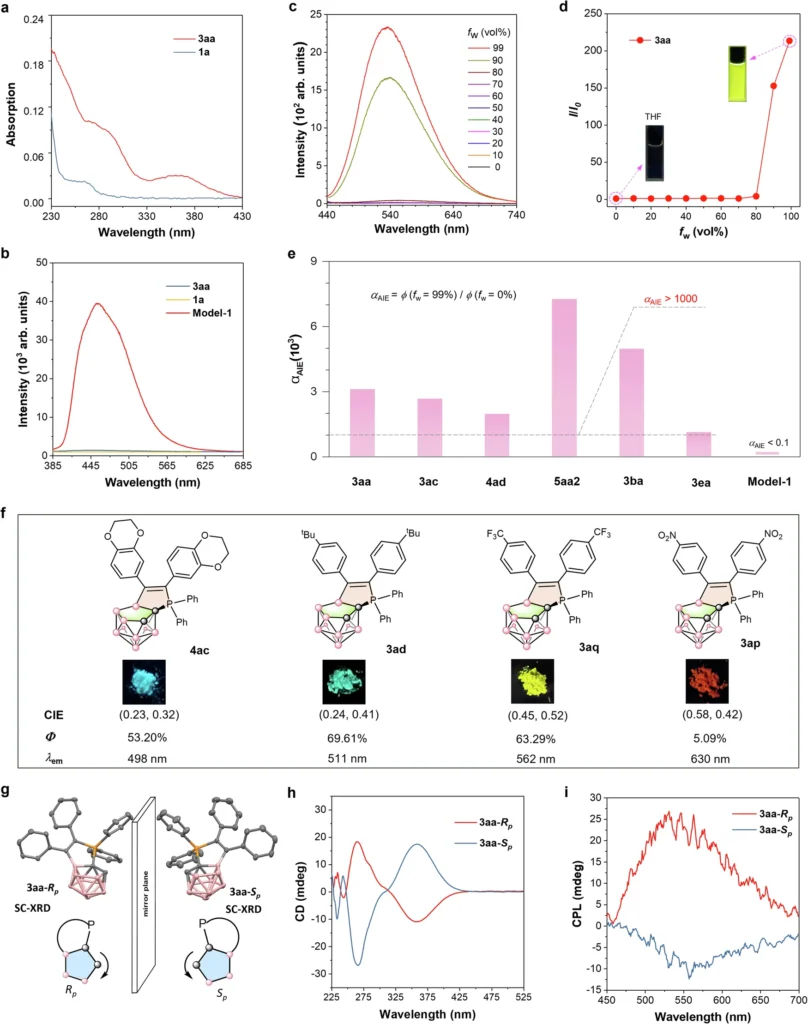
Case Study: Effective Synthesis of a Boron Cluster-Phosphonium Conjugate
Comprehensive Examination of a Practical Synthesis:
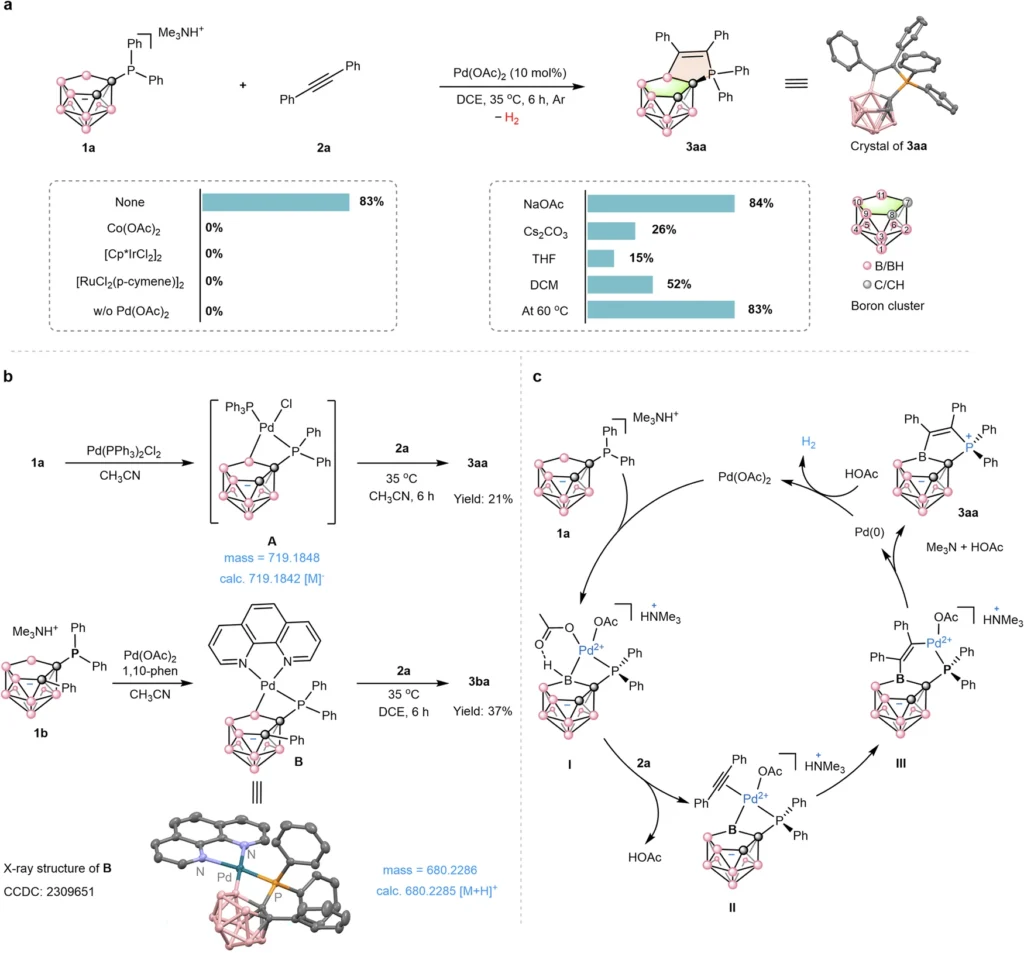
Researchers recently synthesized a boron cluster-phosphonium conjugate using a couple-close construction method. This study looked at a non-classical boron cluster with a cage-like structure. The phosphonium ion, on the other hand, was a substantial ion with four alkyl groups attached to the phosphorus atom.
Recent Research: Principal Observations and Conclusions
The synthesis used a palladium catalyst to create a covalent link between the boron cluster and the phosphonium ion. The reaction occurred efficiently, resulting in the creation of the required conjugate within a few hours.
A key finding of this investigation was the significance of the catalyst in regulating the selectivity of the reaction. In the absence of the catalyst, the reaction progressed sluggishly and produced undesirable byproducts. Regardless, using the palladium catalyst made the reaction more efficient and selective, which led to the creation of the needed conjugate.
Synthetic procedures, obstacles, and resolutions:
Several important steps were needed for the synthesis to happen, such as activating the boron cluster, adding the phosphonium ion, and making a covalent link between the two. The bulky phosphonium ion’s steric hindrance, which hindered its approach to the boron cluster, was a primary hurdle in this synthesis.
To address this difficulty, the researchers employed a linker molecule to establish a separation between the boron cluster and the phosphonium ion. The phosphonium ion was able to reach the boron cluster and form a covalent bond with it without being slowed down by the large number of alkyl groups.
Outcomes and Consequences for Subsequent Research:
The boron cluster-phosphonium conjugate that was made was more stable and reactive, which made it useful for many things, like catalysis and materials research. This synthesis’s success highlights the potential of the couple-close construction technique for developing intricate molecular architectures and also facilitates future endeavors in this domain.
Non-Classical Boron Cluster Conjugates: Characteristics and Benefits
Boron Cluster-Phosphonium Conjugates: Stability and Reactivity
The primary benefits of boron cluster-phosphonium conjugates are their improved stability and reactivity. When you combine the unique bonding patterns of non-classical boron clusters with the stability of phosphonium ions, you get conjugates that are very stable in a wide range of conditions.
These conjugates have an increased reactivity, making them suitable candidates for catalytic processes or other applications requiring high reactivity. The ability to adjust the boron cluster’s reactivity makes these conjugates exceptionally adaptable, allowing for customization for specific purposes.
These Conjugates have distinct electronic characteristics:
Because the electrons in the boron cluster are not all in the same place, boron cluster-phosphonium conjugates have unique electrical properties. The electronic features provide the conjugates with exemplary candidates for application in electronic and photonic devices, necessitating tight control over electronic behavior.
The presence of the phosphonium ion may improve the electronic characteristics of the conjugate, making it more stable or reactive under specific conditions. This makes boron cluster-phosphonium conjugates extremely adaptable, allowing them to be used in a wide range of applications.
Compatibility with a wide range of functional groups and applications:
The primary advantage of boron cluster-phosphonium conjugates is their compatibility with a variety of functional groups. This allows for very simple modifications for specific purposes, ranging from medicine delivery to materials science.
The ability to attach various functional groups to the boron cluster or the phosphonium ion enables researchers to customize the properties of the conjugate for specific purposes. This makes boron cluster-phosphonium conjugates exceptionally flexible, allowing them to be used in a variety of domains.
These Properties Have Advantages for Industrial and Technological Applications:
The distinctive characteristics of boron cluster-phosphonium conjugates make them exemplary candidates for industrial and technological applications. Their improved stability, reactivity, and electrical characteristics render them appropriate for application across diverse domains, including catalysis and electronics.
Their stability and reactivity, along with the ability to change these conjugates for specific uses, make them exceptionally adaptable. Boron cluster-phosphonium conjugates are so well-suited for diverse applications across several industries, including pharmaceuticals and renewable energy.
Ecological and Financial Factors:
Synthesis of Boron Cluster-Phosphonium Conjugates via Green Chemistry:
It is essential to evaluate the environmental implications of synthesizing boron cluster-phosphonium conjugates, as with any chemical process. Researchers are endeavoring to create more environmentally friendly synthetic processes that reduce waste and energy usage. This encompasses the utilization of renewable feedstocks, the advancement of more efficient catalysts, and the application of eco-friendly solvents.
In this domain, the use of recyclable and reusable catalysts is an approach to green chemistry. This diminishes the necessity for substantial amounts of catalysts and mitigates the environmental repercussions of the synthesis. Moreover, researchers are investigating methods to diminish the energy demands of the synthesis process, thereby enhancing its sustainability and environmental compatibility.
Cost-Effectiveness of Couple-Centric Approaches:
The couple-close manufacturing process is exceptionally efficient, rendering it a cost-effective approach for synthesizing boron cluster-phosphonium conjugates. Utilizing a catalyst to expedite the reaction enables researchers to decrease the quantity of initial ingredients needed and reduce the generation of undesirable byproducts. This renders the synthesis more cost-effective and scalable for industrial applications.
Moreover, employing sustainable feedstocks and green chemistry principles can further diminish synthesis costs. By reducing waste and energy usage, researchers can enhance the cost-effectiveness and sustainability of synthesizing boron cluster-phosphonium conjugates.
Scalability in Industrial Applications:
The primary benefit of the couple-close building style is its scalability. This approach is readily scalable for industrial applications, making it suitable for mass production of boron cluster-phosphonium conjugates. By refining reaction conditions and employing effective catalysts, researchers can enhance reaction yield and render the method more cost-effective for industrial applications.
Furthermore, the application of green chemistry concepts, such as catalyst recycling and the use of renewable feedstocks, can improve the synthesis’s scalability. Boron cluster-phosphonium conjugates are a viable choice for a variety of industrial applications.
Opportunities for Waste Minimization and Energy Optimization:
Green chemistry’s primary goal is to reduce waste and energy consumption in chemical operations. Researchers are investigating methods to minimize waste production and enhance energy efficiency in the synthesis of boron cluster-phosphonium conjugates.
An effective strategy for waste reduction is the utilization of more efficient catalysts, which can diminish the generation of undesirable byproducts. Moreover, researchers are investigating methods to recycle and repurpose catalysts, thereby decreasing demand for substantial amounts of raw materials and mitigating waste.
For energy efficiency, researchers are investigating methods to decrease the energy demands of the synthesis process. This includes the use of lower temperatures and pressures, as well as the creation of more efficient reaction conditions that require less energy for completion.
Prospective Developments in Boron Cluster-Phosphonium Chemistry:
Emerging Trends in Boron Cluster and Phosphonium Chemistry:
The subject of boron cluster-phosphonium chemistry is evolving, with various developing themes influencing its future. A notable trend is the advancement of novel synthetic techniques, which enable the formation of more intricate and stable boron cluster-phosphonium conjugates. These strategies assist researchers in expanding the limits of this discipline, resulting in the development of innovative molecules with distinct features.
The investigation of new uses for boron cluster-phosphonium conjugates is a burgeoning topic. Researchers are progressively investigating the possible applications of these conjugates in renewable energy, nanotechnology, and medicine. We expect a rise in demand for novel and more efficient synthesis methodologies as the applications of these conjugates proliferate.
Advancements in Synthetic Methodologies and Catalysis:
A primary catalyst for innovation in boron cluster-phosphonium chemistry is the advancement of novel synthetic methodologies and catalytic systems. Researchers are persistently investigating methods to enhance the efficiency and selectivity of the couple-close building reaction, aiming to produce more intricate and stable conjugates.
A primary goal is to develop novel catalysts that can improve the conjugation process while reducing the generation of undesirable byproducts. These catalysts make the process of making boron cluster-phosphonium conjugates more efficient and scalable for use in industry.
Researchers are investigating novel reaction conditions alongside new catalysts to enhance the efficiency of the synthesis process. This encompasses the use of lower temperatures and pressures, as well as the development of more efficient solvents and reaction media.
Possibility for Interdisciplinary Utilization:
The potential for cross-disciplinary applications is one of the most intriguing facets of boron cluster-phosphonium chemistry. The distinctive characteristics of these conjugates render them exemplary candidates for use across various domains, including medicine and materials research.
Boron cluster-phosphonium conjugates could change how medicines are delivered and how diagnoses are made in medicine. In materials science, they could help make new materials with better properties. These conjugates’ electronic properties make them suitable candidates for use in electrical and photonic devices, necessitating strict control over electronic behavior.
Chemistry’s influence on the future of technology and materials science is significant:
The distinctive characteristics of boron cluster-phosphonium conjugates make them an excellent candidate for a variety of technological applications. From renewable energy to nanotechnology, these conjugates possess the capacity to transform our approach to materials science and chemical synthesis.
Boron cluster-phosphonium conjugates could be used as new catalysts for energy conversion processes in renewable energy, such as turning water into hydrogen and carbon dioxide into useful fuels. In nanotechnology, these conjugates may facilitate the development of novel nanomaterials exhibiting distinctive features, such as improved reactivity or stability.
As more research is done on boron cluster-phosphonium conjugates, this type of chemistry is likely to find more uses. This will have long-lasting effects on the future of technology and materials science.
Final Assessment:
The close connection of non-classical boron cluster-phosphonium conjugates is a big step forward in the field of chemistry. These new compounds present a distinctive amalgamation of stability, reactivity, and electrical characteristics, rendering them exemplary prospects for diverse applications, spanning from medicine to materials science.
Recent improvements in synthetic methods and catalysis have made it easier to make complex boron cluster-phosphonium conjugates. These compounds are quickly becoming useful for many things. As researchers persist in investigating the possibilities of this chemistry, the outlook appears exceptionally promising for boron cluster-phosphonium conjugates.
Frequently Asked Questions:
1). What distinguishes non-classical boron clusters?
Non-classical boron clusters have unique bonding patterns, such as multicenter bonds, which cause them to have unusual electrical and optical properties. These attributes render them exceptionally important for applications in catalysis, materials science, and electronics.
2). In what manner does couple-close construction enhance synthesis?
Couple-close construction makes it easier to carefully control the bonding process, which makes it easier to make complex molecular structures like boron cluster-phosphonium conjugates. This method improves the efficacy and specificity of the synthesis, yielding stable and reactive molecules.
3). What are the practical applications of these conjugates?
Boron cluster-phosphonium conjugates have potential applications in medication delivery, catalysis, material research, and renewable energy. We can utilize them to develop novel materials with improved characteristics or to promote chemical reactions more efficiently.
4). What advantages do these conjugates provide for sustainable chemistry?
Using couple-close construction to make boron cluster-phosphonium conjugates cuts down on waste and energy use, making the process more environmentally friendly. Furthermore, the use of renewable feedstocks and recyclable catalysts reduces the synthesis’ environmental impact.
5). What potential applications can we expect from this research?
Researchers anticipate the emergence of novel applications in domains like nanotechnology, renewable energy, and electronics as boron cluster-phosphonium chemistry research progresses. These conjugates possess the capacity to transform various industries, including pharmaceuticals and materials research.
For more chemistry blogs, visit chemistry Master