Table of Contents
Overview of Covalent Organic Frameworks :
Research on hydrogen production is of paramount importance in the pursuit of clean and renewable energy sources. With the depletion of fossil fuels and growing environmental concerns, there has been a shift in focus towards green energy solutions. Hydrogen is a possible alternative because of its high energy content and lack of carbon emissions when utilized in fuel cells. However, the production of hydrogen effectively and sustainably continues to be a difficulty.
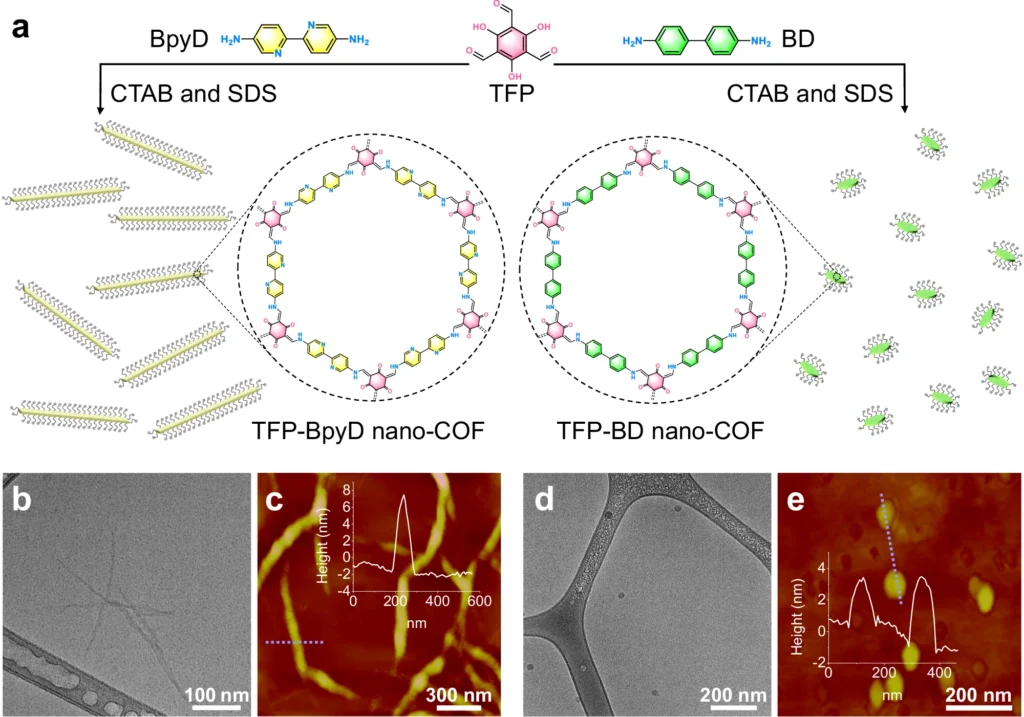
Photocatalysis, the phenomenon of expediting a chemical reaction by utilizing light, has emerged as a promising technique for generating hydrogen. Covalent Organic Frameworks (COFs) have demonstrated considerable potential among the different materials investigated for this objective. In particular, COFs at the nanoscale have attracted attention due to their improved photocatalytic capabilities. This article examines the function of nanoscale COFs in improving the process of photocatalytic hydrogen production. It investigates their distinct characteristics, synthesis techniques, operational processes, and potential future developments. Synthesis of nano-COFs and their morphologies.
Understanding the nature of covalent organic frameworks (COFs):
COFs are a type of crystalline and porous material made up of organic building blocks connected by covalent bonds. These materials possess notable attributes such as exceptional thermal and chemical stability, low density, and a substantial surface area. These qualities render them appropriate for a diverse array of applications, such as gas storage, separation, and catalysis.
Characteristics of Covalent Organic Frameworks structure:
At the molecular scale, one can customize the well-organized and porous structure of COFs. This adjustability enables meticulous regulation of their porosity, surface area, and functional groupings. COFs consist of repeated units that arrange themselves in a regular pattern, resulting in a structured network. This network forms uniform pores that enable the efficient movement of molecules and ions.
Covalent Organic Frameworks Synthesis Techniques:
We have devised various techniques for the production of COFs, each offering distinct benefits and varying degrees of manipulation over the result. The following techniques are included:
Solvothermal synthesis is a process where monomers react in a solvent under high temperatures and pressures, usually in a sealed container. It enables the creation of COFs with a high degree of crystallinity; however, it frequently necessitates extended reaction durations.
Mechanochemical synthesis involves the application of mechanical force, such as grinding, to initiate chemical reactions between the monomers. This technology is free of solvents and is environmentally benign. It can generate Covalent organic frameworks quickly.
Microwave-Assisted Synthesis: This method uses microwave radiation to rapidly heat the reaction mixture, accelerating Covalent organic frameworks production. It reduces response times and improves the crystallinity of the resultant structures.
Room temperature synthesis refers to a technique in which monomers react at normal environmental temperatures, typically with catalysts or special circumstances that encourage the creation of Covalent organic frameworks. This technology is characterized by its excellent energy efficiency and ability to generate Covalent organic frameworks of exceptional quality.
Advantages of COFs in Various Applications:
Carbon-based organic frameworks (COFs) provide numerous benefits that render them well-suited for a wide range of applications:
COFs possess a high surface area, which allows for a greater number of active sites for catalytic processes and improves their ability to adsorb substances.
Tunable Pore Sizes: COFs can finely regulate the diameters of their pores, enabling the targeted adsorption and separation of molecules.
Functional Diversity: COFs can add different functional groups, which makes them better and makes it easier for them to interact selectively with specific molecules.
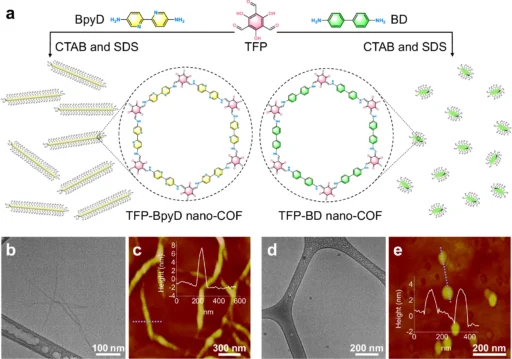
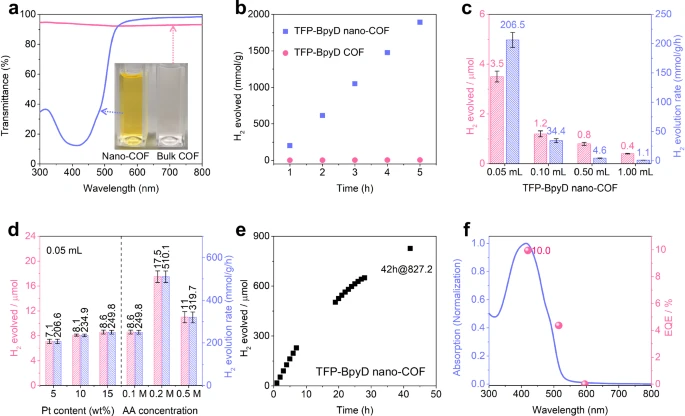
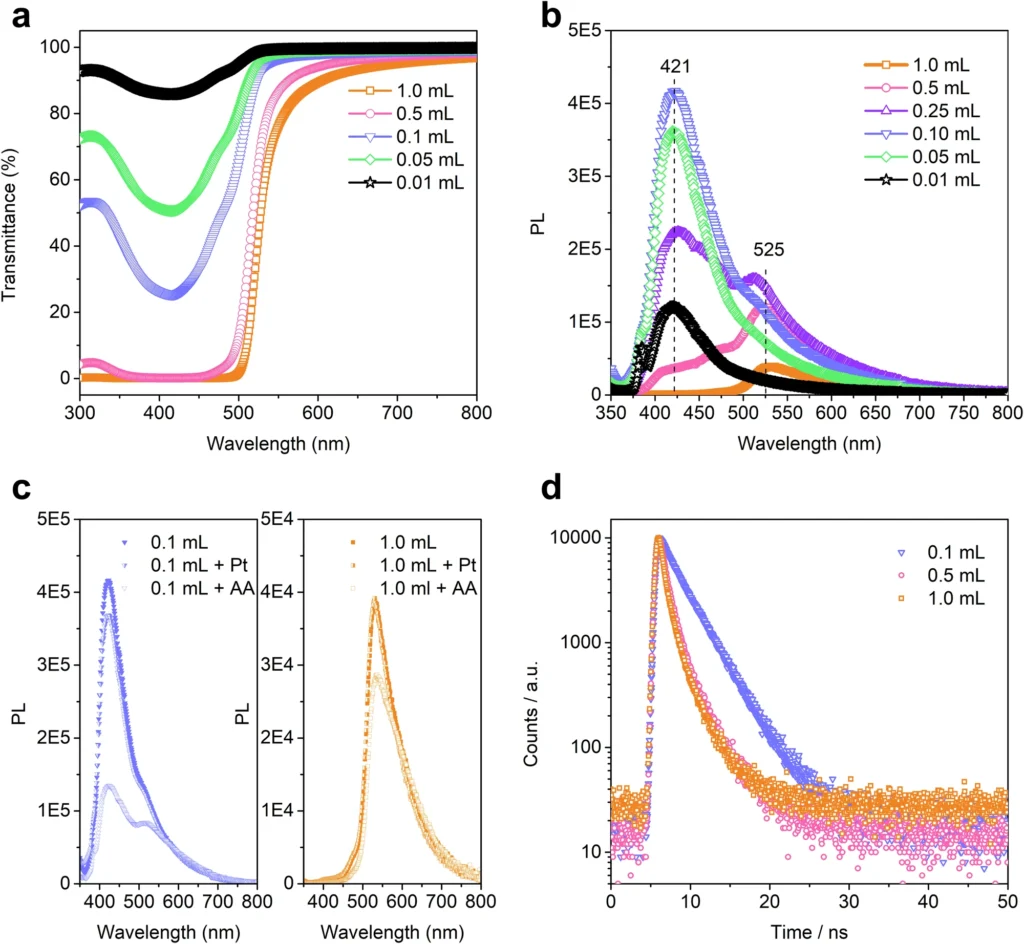
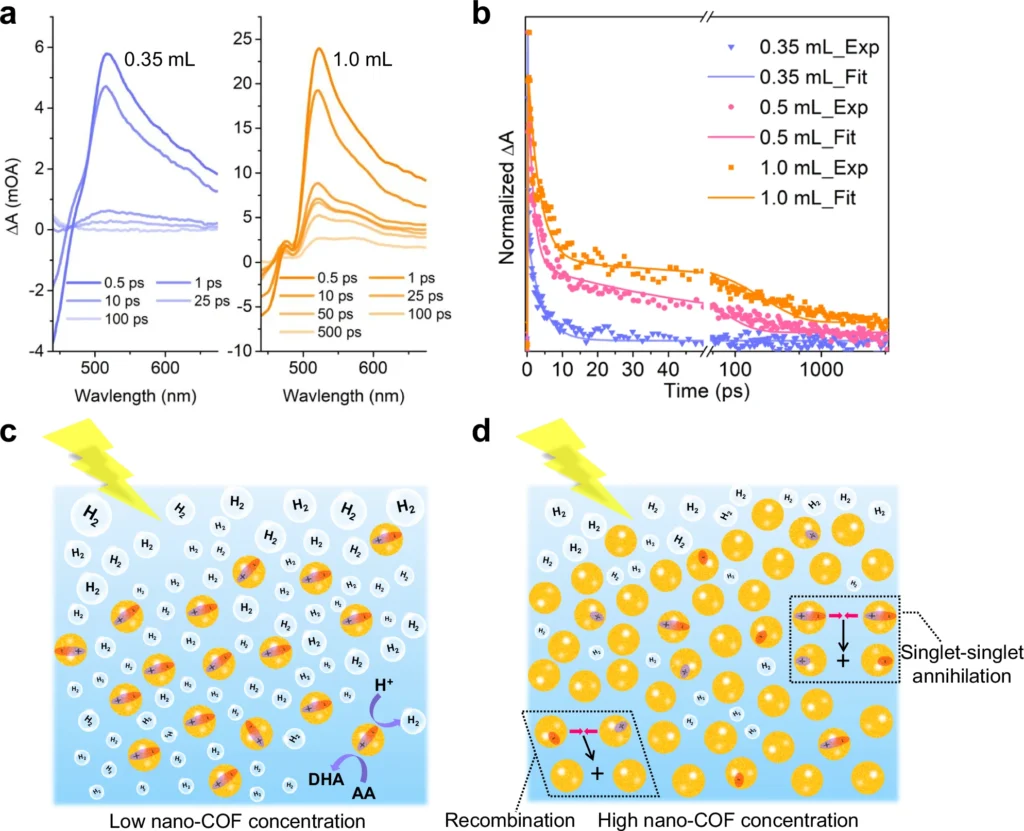
Stability: COFs exhibit exceptional thermal and chemical stability due to their robust covalent bonds, rendering them well-suited for utilization under demanding conditions. Photocatalytic hydrogen evolution for bulk COFs and nano-COFs, and inverse concentration dependence for TFP-BpyD nano-COF.
The Role of Nanoscale COFs in Photocatalysis:
The role of nanoscale covalent organic frameworks (COFs) in photocatalysis is significant.
Nanoscale covalent organic frameworks (COFs) constitute a notable progression in the realm of photocatalysis. By reducing the dimensions of COFs to the nanoscale, their surface area and reactivity are significantly increased, resulting in improved catalytic performance.
Nanoscale COFs have distinct characteristics:
Nanoscale COFs have distinct characteristics that make them highly efficient for photocatalytic applications:
Increased Surface Area-to-Volume Ratio: By reducing the size of the nanoscale, the surface area-to-volume ratio increases, which in turn creates more active sites for catalytic processes.
Enhanced Light Absorption: Nanoscale COFs exhibit enhanced light absorption capabilities, enabling them to capture a greater amount of solar energy for photocatalytic activities.
Faster Charge Separation and Transfer: Because nanoscale COFs are smaller, they can separate and move charge carriers (electrons and holes) more quickly. This means that there are fewer losses from recombination and the photocatalytic process works better.
Advantages of Utilizing Nanoscale COFs in Photocatalysis: Nanoscale COFs used in photocatalysis offer numerous advantages, including:
Nanoscale COFs have higher photocatalytic activity because they have a larger surface area and can absorb light better. This makes the process of making hydrogen more efficient.
Tunability: You can precisely adjust the molecular composition of COFs to maximize their electrical and optical characteristics for specific photocatalytic purposes.
Stability: COFs have exceptional stability due to strong covalent bonds. This enables them to retain their structure and functionality even when exposed to extended periods of light irradiation and harsh chemical conditions.
Photocatalytic hydrogen production mechanisms:
Photocatalysis is the process of utilizing light to expedite a chemical reaction. In hydrogen generation, photocatalysts absorb light energy to stimulate electrons, which then engage in redox processes to produce hydrogen gas from water.
Photocatalysis: An Introduction:
Photocatalysis is based on the stimulation of electrons moving from the valence band to the conduction band of a semiconductor material when it absorbs light. The electron-hole pairs formed are responsible for driving the redox processes that are essential for hydrogen generation.
Procedure for Photocatalytic Hydrogen Generation:
Light Absorption: The photocatalyst absorbs photons, which energizes electrons and propels them to a higher energy level.
Charge separation occurs when the electrons and holes that have been excited are kept apart from each other, thus preventing them from recombining.
Redox reactions involve the reduction of protons to generate hydrogen through the transfer of electrons, as well as the oxidation of water molecules by the movement of holes.
Covalent organic frameworks (COFs) play a crucial role in these processes:
Covalent organic frameworks improve these processes by offering a highly porous structure that allows for effective light absorption and separation of charges. Their adjustable characteristics also enable the enhancement of the redox potential, thereby increasing hydrogen production efficiency. Photophysical properties of TFP-BpyD nano-COF solutions at various concentrations.
Design and synthesize nanoscale COFs for photocatalysis:
When designing Covalent organic frameworks for photocatalysis, it is important to carefully evaluate many parameters, such as the selection of building blocks, the size of the pores, and the functional groups.
Important Factors to Consider While Designing Covalent Organic Frameworks for Photocatalysis:
Choosing suitable monomers and linkers is essential for attaining the intended electrical and optical characteristics. Furthermore, it is crucial to regulate the dimensions and arrangement of the pores to optimize the level of interaction between the COF and the substances undergoing a chemical reaction.
Standard Methods of Synthesis:
Solvothermal methods and mechanochemical methods are common ways to make nanoscale COFs. Solvothermal methods use high temperatures and pressure to make monomers react in a solvent, and mechanochemical methods use mechanical force to start reactions.
Difficulties encountered during the synthesis process:
Although there have been improvements, the process of creating nanoscale COFs with consistent size and excellent crystallinity is still difficult. To fully realize their potential, it is critical to overcome issues such as aggregation and limited scalability.
Nanoscale COFs have improved photocatalytic activity:
Various parameters, including their surface area, light absorption capacity, and charge separation efficiency, influence the photocatalytic efficacy of nanoscale COFs.
Factors affecting photocatalytic efficiency:
Increased surface areas provide a greater number of active sites for catalytic reactions.
Enhancing light absorption leads to an increase in the quantity of electrons that become excited.
Charge separation: The effective separation of electron-hole pairs minimizes losses due to recombination.
Examinations of Increased Activity:
Multiple investigations have proven the exceptional photocatalytic efficiency of nanoscale COFs. For example, using COFs modified with metal nanoparticles or doped with heteroatoms has greatly enhanced the hydrogen production rates.
Comparative Analysis of Photocatalysts:
Covalent organic frameworks (COFs) on a nanoscale level work better than common photocatalysts like metal oxides and sulfides because they are better at absorbing light and moving charges around. Furthermore, their adjustable flexibility offers a significant advantage in optimizing performance.
Nanoscale COFs can be functionalized:
Functionalization is the process of altering the properties of COFs by introducing certain groups or compounds. This technique can greatly enhance the photocatalytic efficiency of COFs.
Functionalization techniques:
A common method is post-synthetic modification, which adds functional groups to COF after it has been made, and in situ functionalization, which adds functional groups during the synthesis process.
Effect of Functionalization on Photocatalytic Efficiency:
Functionalization enhances the ability of COFs to absorb light, facilitate charge transfer, and maintain stability. For instance, the addition of electron-donating groups might augment the reduction potential, while the presence of metal nanoparticles can promote light absorption.
We have attached illustrations of COFs with functional groups:
Examples include the modification of COFs with platinum nanoparticles to enhance hydrogen evolution and the infusion of nitrogen to enhance charge separation. These alterations have resulted in substantial enhancements in photocatalytic efficacy.
Applications of Nanoscale COFs in Hydrogen Production:
Researchers are currently investigating the potential of nanoscale COFs for a range of practical applications, including both independent hydrogen production systems and their integration with already established technologies.
Practical Uses in the Real World:
Solar water-splitting devices commonly use COFs as the primary photocatalyst. Tandem systems also use them, pairing them with other materials to boost overall efficiency.
Compatibility with Current Technologies:
Integrating nanoscale COFs into current hydrogen generation technologies, such as photoelectrochemical cells and reactors, can improve performance and lower expenses.
Prospects for the future:
Researchers are currently focusing on improving the stability, scalability, and efficiency of nanoscale COFs, suggesting a promising future for this technology. We anticipate innovations in synthesis and functionalization to drive further developments. Transient absorption spectra (TAS) for TFP-BpyD nano-COF at different concentrations and mechanistic interpretation.
Obstacles and Constraints:
Despite their considerable potential, nanoscale COFs encounter several obstacles and limits that necessitate resolution.
Present Obstacles in Utilizing Nanoscale COFs:
Stability: It is critical to ensure long-term stability in operational situations.
Scalability: Developing synthesis processes that are both cost-effective and capable of scaling up for practical applications is crucial.
Reproducibility: Ensuring consistent performance across various batches of COFs continues to be a difficulty in terms of reproducibility.
Constraints of COFs in Photocatalysis:
Elaborate Combination: The process of producing superior COFs can be complex and time-consuming.
Materials cost: Certain precursors and agents used in COF production can be expensive.
Restricted Light Absorption: Compared to some inorganic photocatalysts, COFs’ capacity to absorb light is still limited.
Possible resolutions and areas for future investigation:
Future research should prioritize the development of more resilient synthesis processes, the investigation of more affordable and readily available starting materials, and the improvement of COFs’ inherent characteristics through inventive functionalization strategies.
Prospective Developments in COF Research:
The subject of COF research is undergoing rapid evolution, characterized by the emergence of many trends and advances.
Trends in covalent organic frameworks (COF) synthesis and application:
Researchers are currently exploring alternative techniques, like flow chemistry and self-assembly, to produce covalent organic frameworks (COFs) with improved characteristics and on a larger scale. Furthermore, there is a growing interest in hybrid COFs that integrate both organic and inorganic elements.
Advancements in photocatalytic materials:
Notable advancements include the creation of COFs with integrated co-catalysts, COFs with hierarchical structures to boost mass transport, and COFs with enhanced light-harvesting capacities.
Estimates for the Future of Hydrogen Production:
As research advances, we anticipate that nanoscale COFs will significantly influence the shift toward sustainable hydrogen production. We expect their distinctive characteristics and adaptability to lead to the development of novel and more effective hydrogen generation systems.
In conclusion:
Nanoscale covalent organic frameworks have great potential for improving the photocatalytic hydrogen production process. Their unique structural features, adaptability, and potential for functionalization make them ideal for this specific application. Despite the obstacles, continuous research and innovation are leading to the development of more effective and environmentally friendly ways of producing hydrogen. Nanoscale COFs are at the forefront of this promising topic.
Frequently Asked Questions:
1). Covalent bonds bind organic molecules together to form covalent organic frameworks (COFs), a type of crystalline material.
Covalent Organic Frameworks (COFs), composed of light components joined by covalent bonds, are highly stable and tunable crystalline materials. Different applications such as catalysis use these materials with their porous structure.
2). What is the mechanism by which COFs improve the efficiency of photocatalytic hydrogen production?
Carbon-based organic frameworks (COFs) improve the efficiency of photocatalysis hydrogen generation by offering a large surface area for absorbing light, facilitating effective charge separation, and allowing for adjustable characteristics that optimize the redox reactions involved in hydrogen production.
3). What are the difficulties encountered when utilizing nanoscale COFs for hydrogen production?
The challenges include ensuring long-term stability, formulating cost-effective and scalable synthesis procedures, and achieving consistent performance across multiple batches of COFs.
4). What are the anticipated future advances in COF technology?
Future developments could include the refinement of synthesis processes, the use of more cost-effective precursors, the creation of hybrid COFs with improved characteristics, and the introduction of novel functionalization approaches to enhance photocatalytic effectiveness.
5). What is the comparative performance of nanoscale COFs about other photocatalysts?
Nanoscale covalent organic frameworks (COFs) work better than regular photocatalysts because they can absorb light better, transfer charges more efficiently, and be adjusted more easily. This makes them very good at using photocatalysis to make hydrogen.
For more chemistry blogs, visit chemistry Master