Table of Contents
Overview of Effective Photoredox catalysis:
Effective Photoredox catalysis is a cutting-edge method in contemporary chemistry that uses light to initiate chemical reactions. By utilizing photons as an energy source, this technique enables redox processes that were previously challenging or perhaps unattainable under traditional circumstances. Due to its versatility and efficiency, Effective photoredox catalysis finds widespread application in the synthesis of complex compounds, especially in the fields of medicine, materials science, and organic chemistry.
Nevertheless, the effectiveness of photoredox catalysis does not merely rely on the existence of light; rather, the specific wavelength of the light employed significantly influences the results of the reactions. In the past, ultraviolet (UV) and visible light were the best choices for photoredox reactions because they have high energy levels that can effectively stimulate a wide range of photocatalysts. However, these wavelengths also have notable disadvantages, such as their limited ability to penetrate deeply, the higher likelihood of causing damage to delicate materials, and the possibility of side reactions that may negatively affect the quality and quantity of the desired end products.
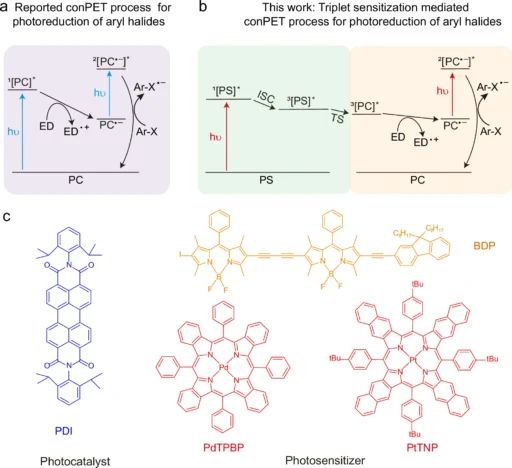
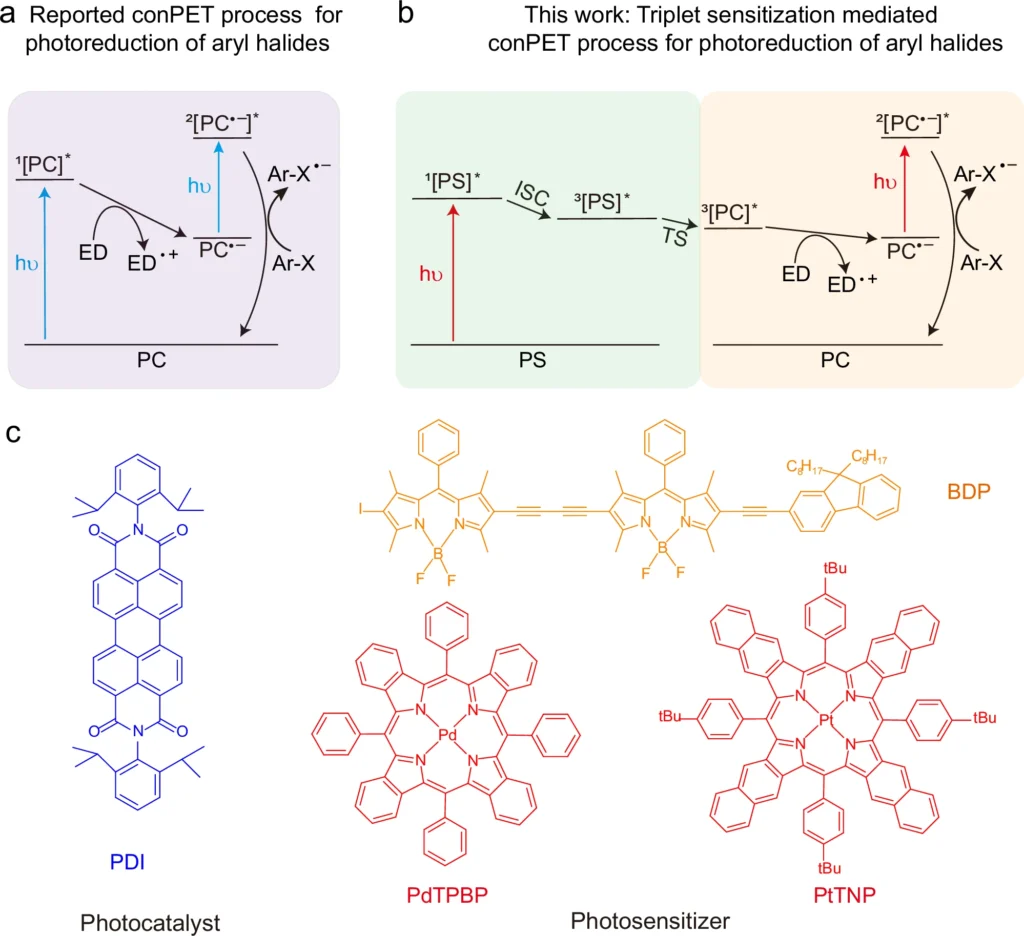
Recently, there has been a shift in emphasis towards longer wavelengths, specifically in the near-infrared (NIR) and red light sections of the electromagnetic spectrum. The longer wavelengths, which have less energy than UV and visible light, have distinct benefits that can improve the efficiency and selectivity of photoredox reactions. This article goes into excellent detail about the scientific ideas behind long-wavelength near-infrared (NIR) and red light-driven sequential photo-induced electron transfer in Effective Photoredox catalysis. It examines the impact of these wavelengths on the field, as well as their role in promoting environmentally friendly and efficient chemical processes. The proposed triplet sensitization-mediated consecutive photoinduced electron transfer (TS-conPET) process for red or NIR light-driven photoreduction of aryl halides
Comprehending for Effective Photoredox Catalysis:
Definition and fundamental principles:
Effective Photoredox catalysis is a mechanism that uses light to activate a photocatalyst, which in turn enables electron transfer between molecules in a redox reaction. The phrase “photoredox” is a combination of the words “photo,” which means light, and “redox,” which refers to reduction-oxidation reactions. In a conventional photoredox cycle, a photocatalyst absorbs light and undergoes excitation to a higher energy level. The photocatalyst’s excited state enables it to transfer electrons to or receive electrons from other molecules, facilitating the redox reaction.
The basic ideas behind Effective photoredox catalysis are based on the photocatalyst’s ability to take in light and change physically, which lets it help move electrons around. This procedure’s efficacy depends on the photocatalyst, light wavelength, and reaction conditions.
The Role of Light in Catalysis:
Light is essential in Effective photoredox catalysis, as it supplies the necessary energy to commence the reaction. The photocatalyst absorbs light, which triggers a transition from its initial state to a higher-energy state. The excited state has higher reactivity and is capable of participating in electron transfer reactions that are not feasible in the ground state.
Shorter wavelengths, such as UV light, have more energy, while longer wavelengths, such as NIR and red light, have less energy. The choice of wavelength is critical, as it directly impacts the efficacy of the electron transfer mechanism and the ultimate result of the reaction.
Benefits of Effective Photoredox Catalysis:
Effective Photoredox catalysis has numerous benefits compared to conventional chemical methods:
Mild Reaction Conditions: Effective Photoredox reactions often take place under mild reaction conditions, characterized by ambient temperature and pressure. This eliminates the need for harsh conditions that may cause unwanted side reactions.
High Selectivity: Effective Photoredox catalysis’s ability to control the reaction environment through light manipulation makes it useful for complex synthetic activities. This is because it yields highly selective products.
Sustainability: Effective Photoredox catalysis is in line with green chemistry concepts because it uses light, a renewable energy source, to minimize waste and decrease the carbon footprint of chemical processes.
Broad Applicability: Effective Photoredox catalysis has a broad range of applications, spanning from small-scale laboratory studies to large-scale industrial processes. This versatility makes it a valuable tool in contemporary chemistry.
The role of wavelength in Effective photoredox catalysis is significant:
The Significance of Wavelength:
The wavelength of light plays a crucial role in Effective photoredox catalysis, as it directly determines the energy level of the photons absorbed by the photocatalyst. The energy should be sufficient to activate the photocatalyst into a reactive state while avoiding severe photodamage or unwanted side reactions.
Photocatalysts exhibit varying absorption spectra, indicating that they efficiently absorb light at various wavelengths. Hence, it is crucial to select the appropriate wavelength for the efficiency and selectivity of the photoredox process.
Typical wavelengths used range from ultraviolet to visible in the electromagnetic spectrum:
Historically, Effective photoredox catalysis has predominantly relied on UV and visible light wavelengths. UV radiation, encompassing wavelengths between 100 and 400 nm, possesses significant energy and readily stimulates numerous photocatalysts. Visible light, ranging from 400 to 700 nm, possesses reduced energy yet is capable of catalyzing a diverse array of reactions.
While these wavelengths are suitable for most applications, they do possess certain limits. Ultraviolet (UV) radiation can induce photodamage to delicate molecules and has a restricted ability to penetrate through reaction media. Although visible light is less harmful, it can still cause adverse reactions and may not reach deep enough to guarantee consistent reaction conditions.
Difficulties associated with short wavelengths:
A major obstacle to utilizing short wavelengths, such as UV light, is the risk of photodamage. It requires intense ultraviolet (UV) photons with enough energy to break chemical bonds in the substrate or photocatalyst. This breaks down the material and creates unwanted byproducts. This poses a significant challenge in the production of intricate organic compounds, as functional group preservation is of utmost importance.
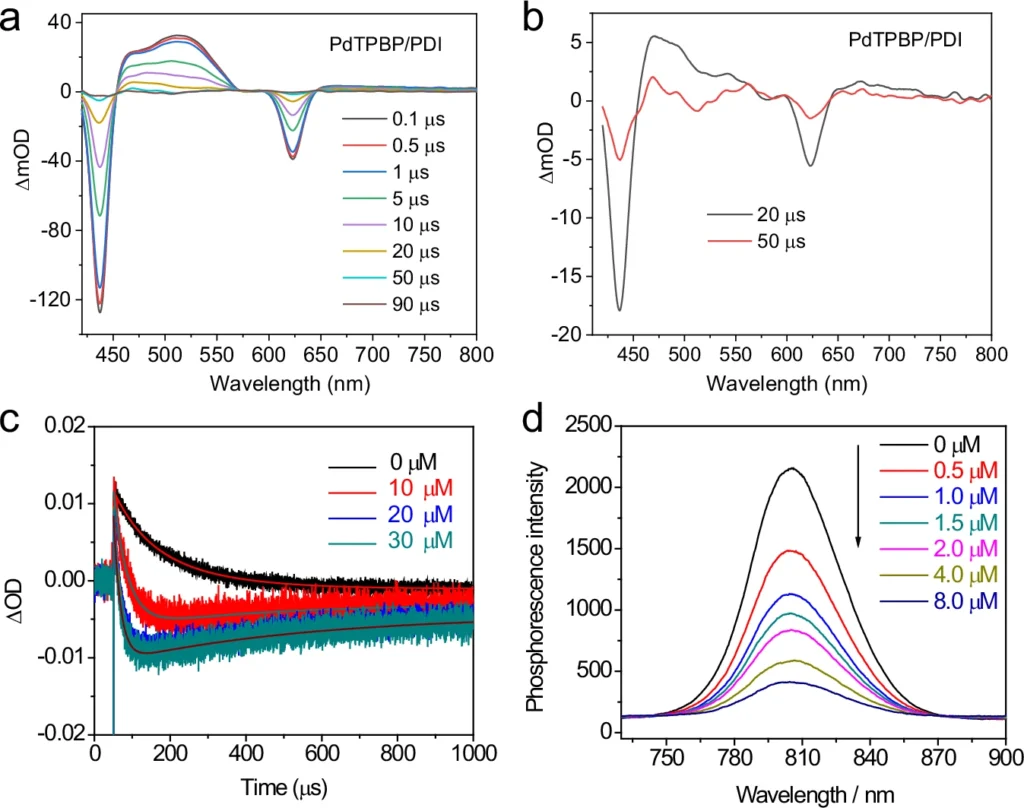
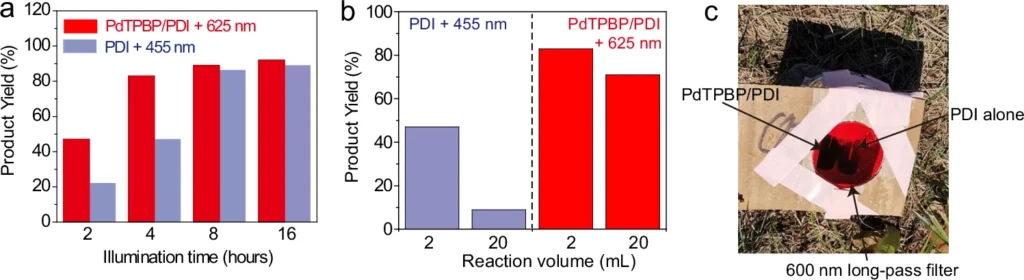
Another challenge is the limited ability of UV and visible light to penetrate deeply. These wavelengths are frequently absorbed or dispersed by the reaction medium, resulting in incomplete reactions or variations within the reaction vessel. The absence of consistency in this regard might undermine the productivity and specificity of the chemical reaction, especially when used on a large scale or in industrial settings. The evidence of the triplet sensitization of PDI by PdTPBP.
An Overview of Long-Wavelength Near-Infrared (NIR) and Red Light:
We discuss the properties of near-infrared (NIR) and red light:
Red light falls within the range of 620 to 750 nm, while near-infrared (NIR) light spans wavelengths between 700 and 2500 nm. The longer wavelengths have lower energy levels in comparison to UV and visible light, which may initially appear to be less advantageous for initiating chemical processes. Nevertheless, NIR and red light exhibit distinct attributes that render them exceptionally valuable in photoredox catalysis.
Near-infrared (NIR) and red light have the notable characteristic of being capable of penetrating materials at greater depths. Unlike UV and visible light, which absorb or disperse rapidly, NIR and red light can easily pass through reaction media with minimal intensity reduction. The significant depth of penetration enables a more consistent and even distribution of light throughout the reaction mixture, which is especially crucial in systems that are either large-scale or have a heterogeneous nature.
Moreover, near-infrared (NIR) and red light’s reduced energy reduces the potential harm from light-induced damage, making these wavelengths ideal for processes involving delicate substrates or intricate molecules. This attribute is particularly advantageous in the pharmaceutical sector, where maintaining the integrity of functional groups is frequently a crucial concern.
Analysis of Penetration Depth and Energy Factors:
The depth to which light may penetrate a reaction medium is a crucial component in determining the effectiveness of photoredox catalysis. Near-infrared (NIR) and red light, due to their longer wavelengths, have more ability to penetrate reaction mixes compared to UV or visible light. The enhanced depth of penetration ensures that light reaches the full volume of the reaction, leading to more consistent and thorough reactions.
Another crucial factor to consider is the decreased energy of near-infrared (NIR) and red light, in addition to their penetration depth. Although these wavelengths possess less energy compared to UV or visible light, they can nonetheless effectively initiate photoredox processes when combined with suitable photocatalysts. Lower energy levels make it less likely that the photocatalyst or substrate will be overexcited. This limits side reactions and makes the process more selective overall.
Applications in a variety of disciplines:
The distinct characteristics of near-infrared (NIR) and red light have introduced novel opportunities in diverse domains of chemistry and materials science. Organic synthesis has employed these wavelengths to generate exceptionally specific reactions that were previously challenging to execute using shorter wavelengths. The specific creation of carbon-carbon bonds, a crucial step in the production of intricate organic compounds, is one application of NIR light.
The pharmaceutical sector employs red light-driven photoredox catalysis to synthesize medicinal compounds with exceptional efficiency and selectivity. The gentle nature of red light is very beneficial for maintaining the fragile functional groups present in numerous medications.
Besides organic synthesis, materials science is investigating NIR and red light to fabricate sophisticated materials with distinctive characteristics. One example is the use of NIR light to trigger polymerization reactions, resulting in materials with improved mechanical strength and thermal stability.
An explanation of sequential photo-induced electron transfer:
An Overview of Electron Transfer Mechanisms:
The phenomenon of electron transfer, which involves the transfer of electrons from a donor molecule to an acceptor molecule, forms the fundamental principle of Effective Photoredox catalysis. The transfer of electrons is the driving force behind the chemical reaction, resulting in the creation of new chemical bonds or the disruption of existing ones.
During a conventional photoredox process, the photocatalyst absorbs light and undergoes excitation to a more energetically favorable state. When the photocatalyst is excited, it can function as either an electron donor or acceptor, enabling the transfer of electrons between the reactants. The electron transfer process can take place either in a single step or in a series of sequential steps, depending on the characteristics of the reaction and the particular photocatalyst employed.
Sequential Analysis of Subsequent Electron Transfer:
When electrons move from one molecule to another, a series of processes are involved in consecutive photo-induced electron transfer. We can divide the process into the following steps:
Photocatalyst Excitation: The photocatalyst absorbs light, typically near-infrared (NIR) or red light, causing it to transition to a higher energy state.
Initial Electron Transfer: During the initial electron transfer, the photocatalyst, while in its excited state, transfers an electron to a molecule near the electron acceptor. This transfer induces a division of charges, with the photocatalyst now in a state of reduction and the acceptor in a state of oxidation.
Consecutive Electron Transfers: After reduction, the photocatalyst can now participate in successive electron transfer processes, transferring electrons to other acceptor molecules. The photocatalyst undergoes a series of successive transfers until it reaches its original condition, thus finishing the catalytic cycle.
Regeneration of the Photocatalyst: After electron transfer steps are completed, photocatalyst regeneration occurs, restoring the photocatalyst to its initial state and enabling its involvement in subsequent catalytic cycles.
One of the main benefits of using NIR and red light in Effective Photoredox catalysis is their ability to facilitate successive electron exchanges. These wavelengths’ reduced energy enables a more regulated and prolonged activation of the photocatalyst, resulting in improved efficiency throughout the catalytic process.
The role of near-infrared (NIR) and red light in improving efficiency is significant:
Near-infrared (NIR) and red light are needed to make Effective Photoredox catalysis work better because they provide a more stable and constant energy source for electron transport. Because these wavelengths have lower energy levels, they are less likely to overstimulate the photocatalyst. This makes it less likely that unwanted side effects will happen or that the photocatalyst itself will break down.
Furthermore, the extensive infiltration of near-infrared (NIR) and red light into the reaction medium ensures uniform lighting of the entire reaction mixture. Uniformity is crucial, especially in large-scale reactions, as uneven light dispersion can result in incomplete reactions or the production of undesirable byproducts.
When these variables come together, NIR and red light work best to make it easier for electrons to move from one process to the next. This makes photoredox reactions more efficient and specific. Red light-driven TS-conPET process is superior than blue light-driven conPET process in view of the photoreduction rate and penetration depth.
The advantages of using long wavelengths inEffective Photoredox catalysis:
Increased penetration depth:
An important benefit of utilizing longer wavelengths, such as near-infrared (NIR) and red light, in Effective Photoredox catalysis, is their increased ability to penetrate deeply. Unlike UV and visible light, which the reaction medium frequently absorbs or disperses, NIR and red light can penetrate deeper into the reaction mixture, ensuring illumination of the entire volume.
Large-scale or heterogeneous reactions benefit greatly from light’s deep penetration because it’s essential to evenly illuminate the reaction mixture. By ensuring adequate illumination of all reactants, near-infrared (NIR) and red light enhances the efficiency and selectivity of the reaction. This results in higher yields and fewer unwanted byproducts.
Minimized photodamage and secondary reactions:
One further significant benefit of employing NIR and red light in Effective Photoredox catalysis is the diminished likelihood of photodamage and unwanted side reactions. The lower energy of these wavelengths makes it less likely that the photocatalyst or substrate will be overstimulated. This keeps molecules from breaking down or making unwanted byproducts.
Preserving the integrity of functional groups is crucial, especially when synthesizing intricate organic compounds. Chemists can utilize near-infrared (NIR) and red light to carry out Effective Photoredox catalysis with more ease, as these conditions are less harsh. This allows for the preservation of the intricate structures of the reactants and ensures that the intended products are produced.
We are enhancing the effectiveness of energy usage and promoting long-term environmental viability:
Using longer wavelengths in Effective Photoredox catalysis additionally enhances energy efficiency and promotes sustainability. NIR to their lower energy requirements compared to UV or high-intensity visible light sources, NIR and red light sources are compatible with the concepts of green chemistry. This results in a reduction in the overall energy consumption of photoredox reactions.
Moreover, the capacity to carry out reactions under less severe conditions diminishes the necessity for excessive heating or cooling, further reducing the energy demands of the process. The energy efficiency of NIR and red light-driven Effective Photoredox catalysis makes it an appealing choice for both academic research and industrial applications, particularly when sustainability and cost-effectiveness are important factors.
Illustrative Cases and Exemplary Instances:
Practical Uses of Near-Infrared (NIR) and Red Light in Effective Photoredox catalysis:
The use of near-infrared (NIR) and red light in Effective photoredox catalysis has resulted in numerous successes in organic synthesis and other domains. An example is the use of NIR light to construct exceptionally specific carbon-carbon bond structures, which is a critical stage in the creation of intricate organic compounds.
In this case, we used near-infrared (NIR) light to trigger a Effective Photoredox catalysis that selectively activated a specific carbon-hydrogen bond, leading to the formation of a new carbon-carbon bond. We conducted the reaction under gentle circumstances, which led to a high level of efficiency and selectivity. This highlights the capability of NIR light in intricate chemical synthesis.
Another example is the use of red light in medication production. Researchers have employed Effective Photoredox catalysis with red light to synthesize medicinal compounds with exceptional accuracy, all while safeguarding the integrity of delicate functional groups. This methodology has demonstrated significant utility in the synthesis of intricate natural compounds, whereas conventional methods frequently lack effectiveness.
Examples of Achievements in Organic Synthesis:
Furthermore, aside from the aforementioned instances, countless other triumphs in organic synthesis effectively demonstrate the potency of near-infrared (NIR) and red light in Effective Photoredox catalysis. For instance, researchers have used NIR light to catalyze targeted oxidation processes, oxidizing specific functional groups while leaving others unaffected. Traditional approaches face challenges in achieving this level of selectivity, thereby highlighting the value of NIR light as a tool for synthesizing complicated organic compounds.
Cross-coupling reactions, involving the formation of a new chemical bond by joining two distinct molecules, have demonstrated the effectiveness of red light. People frequently conduct these reactions under extremely gentle circumstances, exhibiting exceptional efficiency and selectivity, making them highly suitable for the production of medicines and other valuable compounds.
Comparative investigations involving shorter wavelengths:
Comparative studies have shown that photoredox reactions sped up by near-infrared (NIR) and red light often work better than reactions sped up by shorter wavelengths, especially when it comes to the amount of product made and the level of specificity reached. Researchers looked at how UV, visible, and NIR light affected a certain photoredox reaction. They found that NIR light caused the reaction to be most productive and selective, while also lowering the production of unwanted byproducts.
The findings show that near-infrared (NIR) and red light can be useful in photoredox catalysis, especially when simpler methods that use shorter wavelengths don’t work. Near-infrared (NIR) and red light can provide a more regulated and effective energy source, enabling advancements in chemical synthesis and various other domains. Photoreduction of various aryl bromides and chlorides via TS-conPET process with far red light.
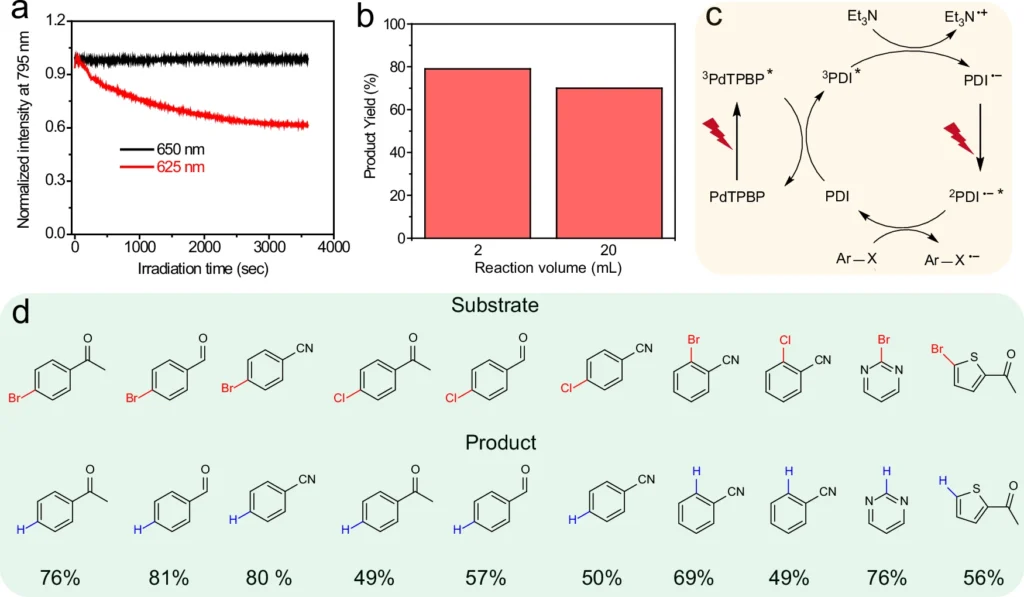
Obstacles and Constraints:
The use of near-infrared (NIR) and red light presented technical challenges:
While NIR and red light present numerous benefits in Effective photoredox catalysis, they also present various technological obstacles that require resolution. A major obstacle lies in developing photocatalysts capable of efficiently absorbing and harnessing these extended wavelengths.
Most conventional photocatalysts are designed to maximize their efficiency in absorbing ultraviolet (UV) or visible light, potentially reducing their ability to absorb near-infrared (NIR) or red light. This can impede the reaction’s efficiency, necessitating higher light intensities or longer reaction times to achieve the intended outcome. Scientists are currently developing novel photocatalysts specifically designed to capture near-infrared (NIR) and red light as a solution to this challenge.
Another technical obstacle lies in the potential complexity of expanding NIR and red-light-driven reactions for industrial applications. Although these wavelengths are efficient in laboratory settings on a small scale, implementing these technologies for large-scale industrial production necessitates overcoming substantial technological and economic obstacles. For example, the costs associated with NIR and red light sources may exceed those of conventional UV or visible light sources, and the development of reactors capable of consistently illuminating large reaction volumes with these wavelengths can be complex.
Possible Constraints in Specific Reactions:
Although NIR and red light have numerous benefits, they may not be appropriate for every photoredox process. Occasionally, the relatively low energy of these wavelengths may not be adequate to stimulate certain photocatalysts or facilitate particular processes. Situations requiring extremely high-energy activation may restrict the utilization of NIR and red light, thereby limiting the range of reactions that can occur.
Furthermore, researchers are actively developing photocatalysts specifically tailored to absorb near-infrared (NIR) and red light. Although there have been notable advancements in this field, the selection of photocatalysts that are efficient at longer wavelengths is still somewhat restricted in comparison to those specifically designed for UV and visible light. This constraint can limit the range of reactions that near-infrared (NIR) and red light-driven photoredox catalysis can efficiently conduct.
The challenge lies in managing a growing workload while cutting costs:
The financial burden associated with these procedures is a major obstacle to expanding the use of NIR and red light-driven photoredox reactions for industrial purposes. Although NIR and red light sources are more accessible, they still tend to be pricier compared to conventional UV or visible light sources. Furthermore, developing high-capacity reactors capable of effectively harnessing these wavelengths can be intricate and expensive.
Current research focuses on developing more cost-effective and scalable solutions for photoredox catalysis using near-infrared (NIR) and red light to address these challenges. This includes the development of more efficient light sources, the creation of reactors that can achieve uniform light dispersion, and the creation of innovative photocatalysts specifically designed for these particular wavelengths.
Advancements in Photoredox Catalysis Technology:
Advancements in Catalyst Design:
The field of photoredox catalysis is progressing rapidly, with many technological advancements aiding in the resolution of the difficulties related to the use of near-infrared (NIR) and red light. The development of novel photocatalysts, specifically tailored to efficiently absorb longer wavelengths of light, is a key area of innovation.
These newly developed catalysts frequently utilize materials that exhibit high absorption in the near-infrared (NIR) and red-light areas of the electromagnetic spectrum. Examples of such materials include certain metal complexes, organic dyes, and semiconductor materials. Scientists can make photocatalysts that respond strongly and only to near-infrared (NIR) and red light by changing the electrical properties of these substances. This makes photoredox reactions more efficient and selective.
Furthermore, there has been a notable advancement in the creation of heterogeneous photocatalysts that can be effortlessly retrieved and employed repeatedly in several reaction cycles. People frequently place these catalysts on solid substrates like silica or metal oxides to enhance their durability and adapt them for industrial settings.
Progress in Illumination Sources and Equipment:
In addition to progress in catalyst design, there have been notable advancements in light-source technology. Currently, there are advanced light-emitting devices, such as high-power LEDs and lasers, that can emit near-infrared (NIR) and red light with both high intensity and precision. Compared to conventional light sources, these devices have numerous benefits, such as the ability to accurately regulate the wavelength and intensity of the light, as well as the ability to concentrate the light on specific regions of the reaction vessel.
Furthermore, significant advancements have been made in the development of photoreactors, which are used to implement photoredox reactions on a larger scale. These reactors frequently come with specialized light delivery systems that provide consistent illumination of the reaction mixture, even in substantial quantities. Ensuring uniform reaction conditions over the whole reaction volume is crucial in industrial applications, especially for generating high yields and maintaining product quality.
Future advancements in near-infrared (NIR) and red light catalysis are underway:
In the realm of NIR and red light-driven photoredox catalysis, numerous captivating trends lie ahead. An area of research with great potential is the advancement of photocatalysts that can function under lower energy conditions, such as far-infrared (FIR) or microwave light. These catalysts have the potential to enhance the energy efficiency and sustainability of chemical processes.
Another developing pattern is the combination of photoredox catalysis with other cutting-edge technologies, such as flow chemistry and machine learning. Flow chemistry, a method that utilizes a continuous flow of reactants through a reactor instead of performing reactions in batches, provides numerous benefits in terms of scalability and process control. Researchers can enhance the efficiency and scalability of reactions by integrating photoredox catalysis with flow chemistry. This combination also allows for the possibility of optimizing reactions in real time using machine learning algorithms. Demonstration of the versatility of this TS-conPET strategy.
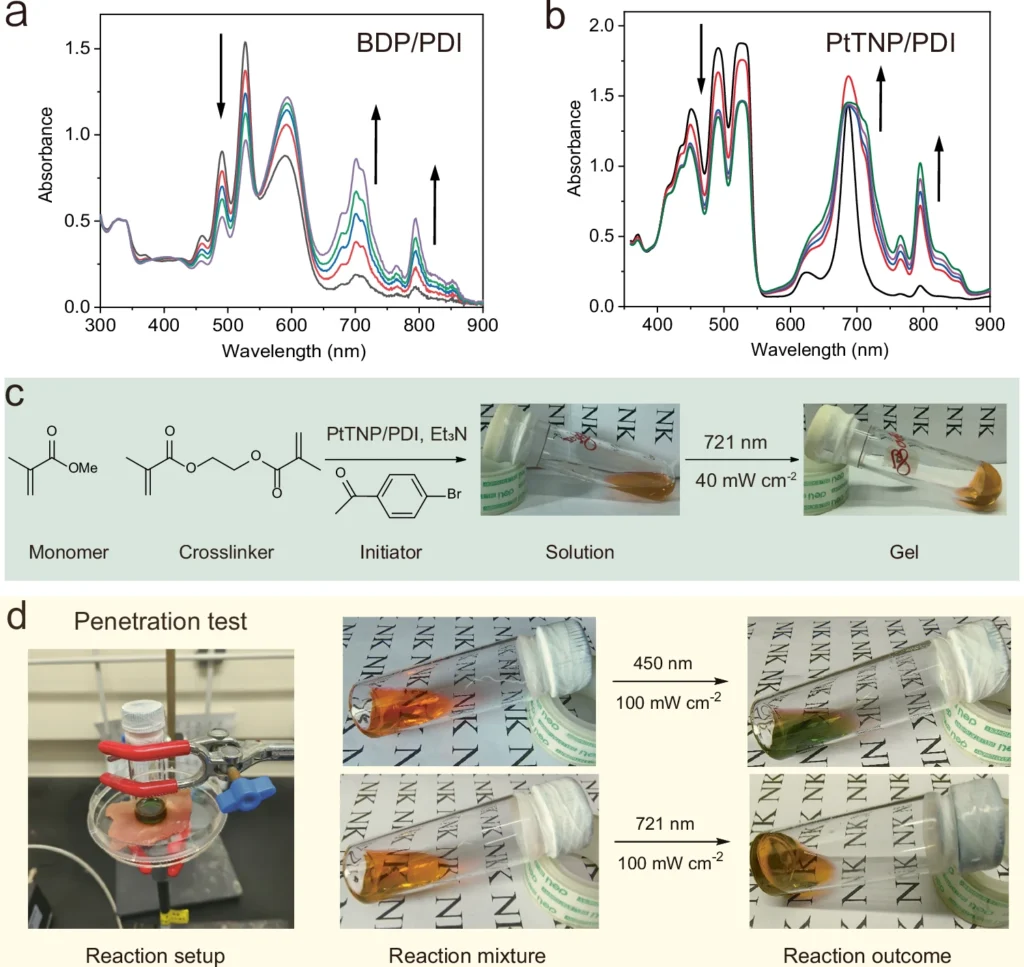
Environmental and Industrial Impact:
There are factors to consider in relation to sustainability:
An important benefit of NIR and red light-driven Effective photoredox catalysis is its capacity to enhance the sustainability of chemical processes. Effective Photoredox catalysis uses light as an energy source, reducing the need for external heating or cooling while also reducing overall energy consumption. This is consistent with the principles of green chemistry, which seek to minimize waste and decrease the environmental consequences of chemical manufacturing.
Furthermore, the capacity to conduct reactions under gentle conditions diminishes the necessity for aggressive chemical reagents, which have the potential to produce dangerous waste. Ensuring proper disposal of toxic byproducts is of utmost importance, especially in the pharmaceutical industry. Utilizing near-infrared (NIR) and red light-driven photoredox catalysis allows for substantial quantities of the intended products while minimizing waste, thereby improving the process’s environmental sustainability.
The advantages of green chemistry:
Green chemistry is a rapidly expanding field that is gaining significance as global industries strive to minimize their ecological footprint and embrace more sustainable methodologies. The use of near-infrared (NIR) and red light-driven photoredox catalysis provides numerous advantages, such as reduced energy consumption, waste generation, and the use of renewable energy sources.
For instance, we can use solar energy to drive photoredox reactions, providing a renewable and sustainable light source. Areas with abundant sunlight can particularly benefit from solar-powered photoredox catalysis, enabling the production of chemicals and materials with minimal carbon emissions.
Furthermore, NIR and red light-driven photoredox catalysis, in addition to their positive impact on the environment, can also enhance economic sustainability. This approach can decrease the requirement for costly reagents and energy-intensive procedures, thereby reducing the cost of chemical production. As a result, it becomes more affordable for a broader range of industries.
Industrial Applications and Economic Impact:
The industrial uses of near-infrared (NIR) and red light-driven photoredox catalysis are extensive and diverse. The pharmaceutical industry currently employs this approach to efficiently and accurately synthesize intricate drug molecules. Conducting reactions at low temperatures is highly beneficial for maintaining the stability of delicate functional groups, which is crucial in the production of numerous pharmaceutical compounds.
Materials science employs near-infrared (NIR) and red light-driven photoredox catalysis to fabricate sophisticated materials with distinctive attributes such as improved mechanical robustness, thermal endurance, and electrical conductance. Various industries, including electronics, aerospace, and renewable energy, can utilize these materials.
The economic consequences of photoredox catalysis driven by NIR and red light are also substantial. This approach has the potential to reduce the energy and material costs associated with chemical production, thereby lowering the overall cost of manufacturing. Consequently, it can enhance competitiveness in the global market. Moreover, the capacity to manufacture chemicals and materials with minimal waste and environmental consequences can assist enterprises in complying with progressively strict environmental requirements, thereby avoiding expensive penalties and enhancing their public reputation.
Comparative Analysis: Near-Infrared (NIR) and Red Light vs. Conventional Methods
Optimizing productivity and achieving desired outcomes:
Using near-infrared (NIR) and red light for photoredox catalysis has many important advantages over traditional catalytic methods in terms of efficiency and effectiveness. One of the main advantages is the ability to carry out reactions under less severe conditions, thereby reducing the likelihood of unwanted side reactions and enhancing the process’s selectivity. Ensuring the preservation of functional groups is of utmost importance, especially in the synthesis of intricate molecules.
Furthermore, the use of NIR and red light can enhance the reaction’s efficiency by guaranteeing consistent illumination of the reaction mixture. In large-scale or heterogeneous reactions, ensuring uniform light distribution is crucial in reactions that are large-scale or heterogeneous, as uneven distribution can result in incomplete reactions or the production of undesired byproducts. By ensuring adequate exposure of all reactants to light, NIR and red light-driven photoredox catalysis can enhance yields and selectivity, surpassing the effectiveness of conventional approaches.
Efficiency and feasibility:
Another crucial factor to consider is the cost-effectiveness of near-infrared (NIR) and red-light-driven photoredox catalysis. Although the upfront cost of specialized light sources and photocatalysts may exceed that of traditional methods, the long-term cost savings in energy and materials can make these processes more cost-effective over time. Furthermore, the ability to carry out reactions under less severe conditions may reduce the need for costly reagents and waste, thereby reducing the overall cost of production.
The creation of novel light sources and photoreactors specifically tailored for NIR and red light-driven photoredox catalysis further enhances its effectiveness. These improvements in technology have made it easier to use near-infrared (NIR) and red light-driven photoredox catalysis on a large scale, which makes it a good choice for many uses.
Comparisons of cases:
To demonstrate the benefits of near-infrared (NIR) and red light-driven photoredox catalysis, we will examine many case comparisons with conventional techniques. One example involved a pharmaceutical company successfully creating a complicated medicinal molecule using red light-driven photoredox catalysis. This method resulted in higher yields and improved selectivity compared to conventional thermal procedures. The red light’s gentle conditions aided in maintaining the integrity of the functional groups, leading to a superior product with reduced contaminants.
Another example was the use of NIR light by a materials science company to stimulate a polymerization reaction, resulting in the production of a material with improved mechanical strength and thermal stability. The NIR light-driven reaction exhibited higher energy efficiency and resulted in a more homogeneous material with enhanced characteristics, in contrast to conventional approaches.
These examples show how near-infrared (NIR) and red light-driven photoredox catalysis can produce great results in a wide range of situations, from pharmaceuticals to advanced materials.
Potential opportunities for the future:
Advancing Studies and Innovation:
The prospects for NIR and red light-driven photoredox catalysis are promising, as continuous research and development facilitate the emergence of novel applications and advancements. An area of research that is particularly captivating is the advancement of novel photocatalysts capable of absorbing and harnessing longer wavelengths, including far-infrared (FIR) light. These catalysts have the potential to enhance energy efficiency and sustainability in chemical processes, hence expanding opportunities in green chemistry and renewable energy.
One further field of developing investigation is combining photoredox catalysis with other cutting-edge technologies, like flow chemistry, machine learning, and artificial intelligence. By combining these technologies, researchers can produce more efficient and scalable reactions with the possibility of real-time optimization and process control.
Potential new applications:
As NIR and red light-driven photoredox catalysis continue to advance, new applications are likely to arise in a wide range of sectors. This strategy in the pharmaceutical business could yield novel medications with enhanced efficacy and reduced adverse effects, especially in the treatment of complex diseases like cancer and neurodegenerative disorders.
NIR and red light-driven photoredox catalysis in the field of materials science could generate new materials with unique features like self-healing capabilities, better conductivity, or improved thermal stability. These materials potentially have a wide range of uses in areas such as electronics, aerospace, and renewable energy.
Beyond these sectors, environmental remediation could also utilize NIR and red light-driven photoredox catalysis to break down contaminants or synthesize environmentally acceptable compounds. The ability to accomplish reactions under mild conditions and with low waste makes this technique is particularly well-suited to sustainable and green chemical applications.
The Long-Term Vision for Photoredox Catalysis:
Looking to the long term, the vision for NIR and red light-driven photoredox catalysis is one of continual innovation and expansion. We anticipate this approach to become increasingly efficient, adaptable, and widely adopted as new photocatalysts, light sources, and reactor designs develop. The possibility of merging photoredox catalysis with other modern technologies, such as machine learning and artificial intelligence, could significantly boost its capabilities and open up new vistas in chemical synthesis and materials research.
Ultimately, the goal is to make NIR and red light-driven photoredox catalysis a mainstream technology in the chemical industry, with widespread applications in medicines, materials research, environmental remediation, and beyond. By continuing to push the frontiers of what is feasible with this method, researchers and industry professionals may help establish a more sustainable and inventive future for chemistry.
Conclusion:
In conclusion, Effective photoredox catalysis using long-wavelength near-infrared and red light to drive successive photo-induced electron transfer is a powerful and promising method. By utilizing the unique features of these longer wavelengths, researchers and industry professionals can produce extremely effective and sustainable chemical transformations. NIR and red light-driven photoredox catalysis is a good choice for many uses, from pharmaceuticals to materials research, because it can be used in less harsh conditions, with a lower chance of photodamage and better selectivity.
As technology continues to progress, the potential applications of NIR and red light in photoredox catalysis are nearly unlimited, making this an intriguing area of study with enormous implications for the future of chemistry. By continuing to investigate and improve this technique, we can uncover new possibilities in chemical synthesis, materials science, and environmental remediation, paving the way for a more sustainable and innovative future.
FAQs:
1). What is an Effective photoredox catalysis?
Effective Photoredox catalysis utilizes light to trigger redox reactions, facilitating the transfer of electrons between molecules under benign conditions. Organic synthesis, materials science, and medicine, among other domains, widely use this method.
2). Why are NIR and red light important in catalysis?
NIR and red light offer distinct advantages, such as deeper penetration into reaction media, less photodamage, and greater energy efficiency, making them excellent for complicated and delicate chemical processes. These longer wavelengths enable more regulated and efficient photoredox reactions, leading to greater yields and selectivity.
3). In photoredox reactions, how does electron transfer work?
In photoredox reactions, light stimulates a photocatalyst, which then facilitates the transport of electrons between molecules, driving the chemical process ahead. This process can entail successive electron transfers, in which many phases occur, increasing the overall efficiency of the reaction.
4). What are the main challenges to using long wavelengths?
The key hurdles involve creating effective photocatalysts that can absorb NIR and red light and scaling up these reactions for industrial usage. Additionally, the availability of photocatalysts specifically tuned for these longer wavelengths is currently somewhat restricted, which can limit the types of reactions that can be effectively carried out.
5). What are the future trends in Effective photoredox catalysis?
Future trends include the creation of new photocatalysts, breakthroughs in light source technology, and the integration of photoredox catalysis with other modern technologies such as flow chemistry and machine learning. These developments are likely to broaden the applicability of photoredox catalysis to new domains, including renewable energy, environmental remediation, and advanced material synthesis.
For more chemistry blogs, visit chemistry Master