Table of Contents
Overview of Fatty Acid Synthase:
Fatty acid synthase (FAS) is a key player in the intricate realm of metabolic processes. It plays a significant part in the production of fatty acids, which are essential for building cellular membranes and storing energy. While Fatty acid synthase (FAS) is primarily known for its role in fatty acid production, recent biotechnology advancements have revealed the potential of its constituent, malonyl/acetyl-transferase (MAT), as a powerful tool for manipulating complex compounds known as polyketides. These naturally occurring chemicals serve as the foundation for a wide range of medications, such as antibiotics and anticancer drugs.
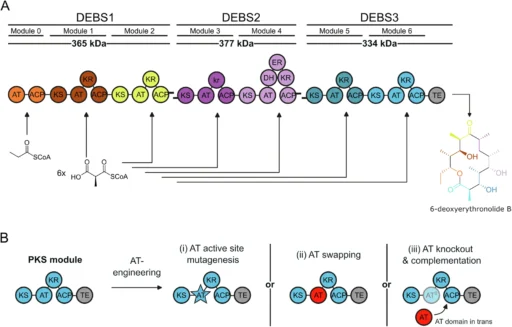
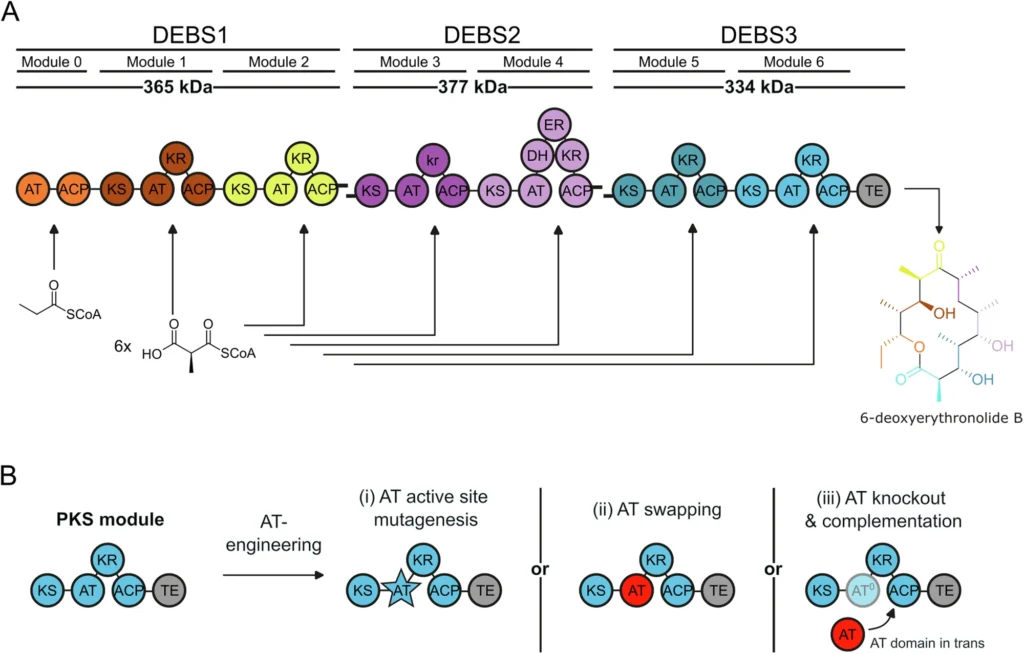
This article examines the role of MAT derived from murine (mouse) Fatty acid synthase (FAS) in polyketide framework modification and manipulation. We will investigate the distinctive characteristics of this enzyme, including its ability to catalyze several reactions and utilize this trait to produce a wide range of polyketide structures. This will facilitate the development of new drugs and advancements in biotechnology. Modular architecture of DEBS PKS and AT engineering approaches.
Analyzing the Mechanism of Fatty Acid Synthase (FAS):
We will discuss the structure and function of FAS (fatty acid synthase):
Fatty Acid Synthase is a substantial, versatile enzyme complex that is responsible for producing long-chain fatty acids from acetyl-CoA and malonyl-CoA. In mammals, Fatty acid synthase (FAS) works as a dimer, with several catalytic domains in each monomer. These domains help the condensation, reduction, dehydration, and final reduction of substrates to make palmitate, a 16-carbon saturated fatty acid.
Constituents of the Murine Fatty acid synthase (FAS) Complex:
The Fatty acid synthase (FAS) complex in murine models consists of many domains, each serving a distinct purpose. Three parts were talked about: the acyl carrier protein (ACP), the β-ketoacyl synthase (KS), and the malonyl/acetyl-transferase (MAT) domain. Moving malonyl and acetyl groups from their CoA derivatives to the ACP is made easier by the MAT domain. This is a very important step in the elongation of fatty acids.
The role of medication-assisted treatment (MAT) in fetal alcohol syndrome Fatty acid synthase (FAS):
The MAT domain is important in the Fatty acid synthase (FAS) cycle because it facilitates the initial steps of transferring the acyl groups onto the ACP. The loading process prepares the fatty acid chain for future extension and alteration. If the MAT enzyme lacks transferase activity, the Fatty acid synthase (FAS) complex would be incapable of initiating the synthesis process.
We are conducting a comparative analysis of fetal alcohol syndrome Fatty acid synthase (FAS) in mice and other species:
Although the fundamental role of Fatty acid synthase (FAS) remains consistent across different species, there can be variations in the structural arrangement and control mechanisms of this enzyme. Researchers have extensively researched murine Fatty acid synthase (FAS), similar to its human counterpart, to understand its role in creatures of greater complexity. Many people are interested in the MAT domain in murine Fatty acid synthase (FAS) because it is very selective and good at helping transfer reactions happen. These are qualities that are very useful in biotechnology.
Significance of Fatty acid synthase (FAS) in Evolution:
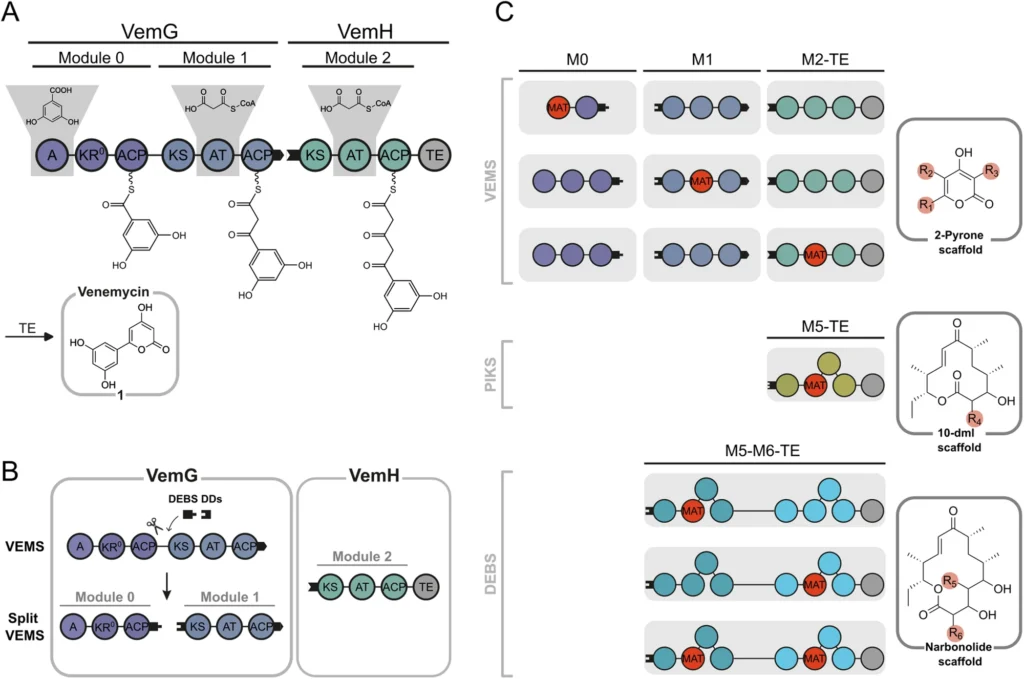
The conservation of Fatty acid synthase (FAS) across various organisms underscores its crucial role in cellular metabolism. Nonetheless, researchers have been able to use the slight disparities in enzyme structure and function among different species to their advantage in targeted biotechnological endeavors, such as the manipulation of polyketide frameworks. Module and domain organization of VEMS split VEMS, and AT/MAT-swapping in modules of VEMS, PIKS, and DEBS.
Malonyl/Acetyl Transferase (MAT) is an enzyme involved in the process of synthesizing fatty acids:
Methionine adenosyltransferase (MAT) operates through a biochemical mechanism:
The MAT enzyme does its job by moving malonyl and acetyl groups from CoA derivatives to the Fatty acid synthase (FAS) complex’s ACP domain. The transfer mentioned here is an essential stage in the fatty acid synthesis cycle, as it supplies the acyl groups required for the subsequent steps of elongating the chain. The active region of the enzyme is highly specialized to efficiently recognize and catalyze these unique reactions.
The role of MAT is crucial in the transfer of Acetyl and Malonyl groups:
MAT’s primary function is to facilitate the transacylation processes that transfer acyl groups onto the ACP. The active site shape of the enzyme effectively accommodates acetyl and malonyl groups, leading to their selectivity. Although MAT has a defined scope, it has demonstrated an impressive capacity to accommodate and transport different substrates in controlled settings. This characteristic is critical for its application in polyketide engineering.
The MAT (Mating Type) system possesses both specificity and promiscuity:
The MAT enzyme is known for being promiscuous, which means it can interact with and transfer acyl groups that are not normally found on its substrates. Because it can incorporate new acyl groups into polyketide scaffolds to create a wide range of structural variations, MAT’s property makes it an indispensable tool in metabolic engineering.
MAT, or Materiality Assessment Tool, is a component of the whole Financial Accounting Standards Fatty acid synthase (FAS) process:
MAT collaborates with other domains within the Fatty acid synthase (FAS) complex to guarantee the accurate elongation and modification of fatty acids. The primary function of this domain is to provide the essential components, while the other domains are responsible for extending the chain and making future alterations. The interaction between these domains emphasizes the intricate nature of the Fatty acid synthase (FAS) system and the significance of MAT in preserving its functionality.
Comparisons of Malonyl and Acetyl Transfer via MAT:
While MAT can transfer both malonyl and acetyl groups, the enzyme displays nuanced variations in how it handles these substrates. The variations stem from the enzyme’s active site and its interactions with the substrates. Understanding these subtle distinctions is critical to effectively using MAT for the creation of various polyketide structures. Split VEMS with a promiscuous loading module.
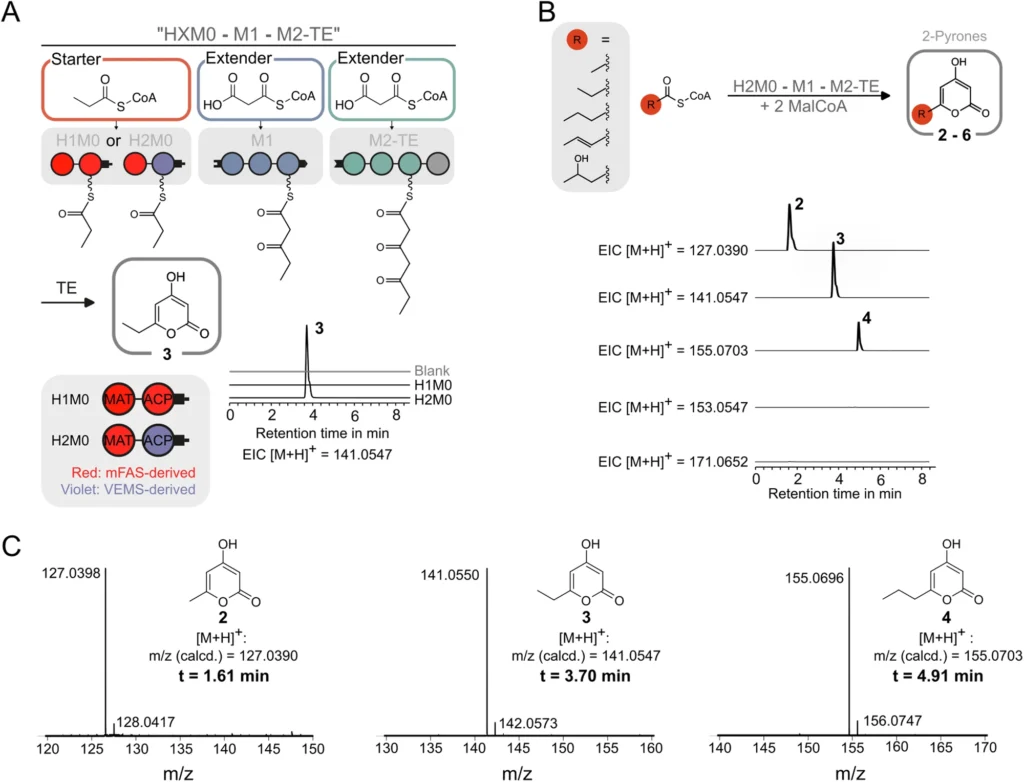
Polyketides are highly adaptable compounds found in nature:
Polyketides: An Overview
Polyketides are a group of natural substances known for their intricate and diverse structures. They are synthesized by polyketide synthases (PKS), which are modular enzyme complexes similar to Fatty acid synthase (FAS). Polyketides form the fundamental structure of several pharmaceuticals, encompassing antibiotics, immunosuppressants, and anticancer medications.
Polyketides exhibit a wide range of structural diversity:
Polyketides can have a lot of different structures because PKS enzymes are modular, which means they can put together different building blocks in several different ways. The capacity to incorporate various functional groups augments the diversity, resulting in a broad range of chemical structures with distinct biological functions.
Polyketides hold great importance in the domains of biology and pharmaceuticals:
The powerful and significant biological properties of polyketides make them highly prized in the field of medicine. Polyketides include antibiotics like erythromycin and anticancer medicines like doxorubicin. The capacity to manipulate these molecules provides opportunities for creating medications with improved effectiveness and diminished adverse reactions.
Difficulties in Polyketide Synthesis:
The complexity of the biochemical pathways involved is a significant challenge in polyketide production. To create new molecules through these pathways, a thorough comprehension of the enzymes involved is necessary, particularly the MAT domain, which has a crucial function in shaping the final product’s structure.
Engineering Polyketide synthases (PKS) alter the functions of these enzymes:
Scientific advancements in genetic engineering and synthetic biology have facilitated the alteration of PKS enzymes, enabling the creation of new and unique polyketides. Researchers can create new molecules with specific features by modifying the domains involved in chain elongation and modification, such as the MAT domain. Promiscuous assembly line with substrate-dependent starting points.
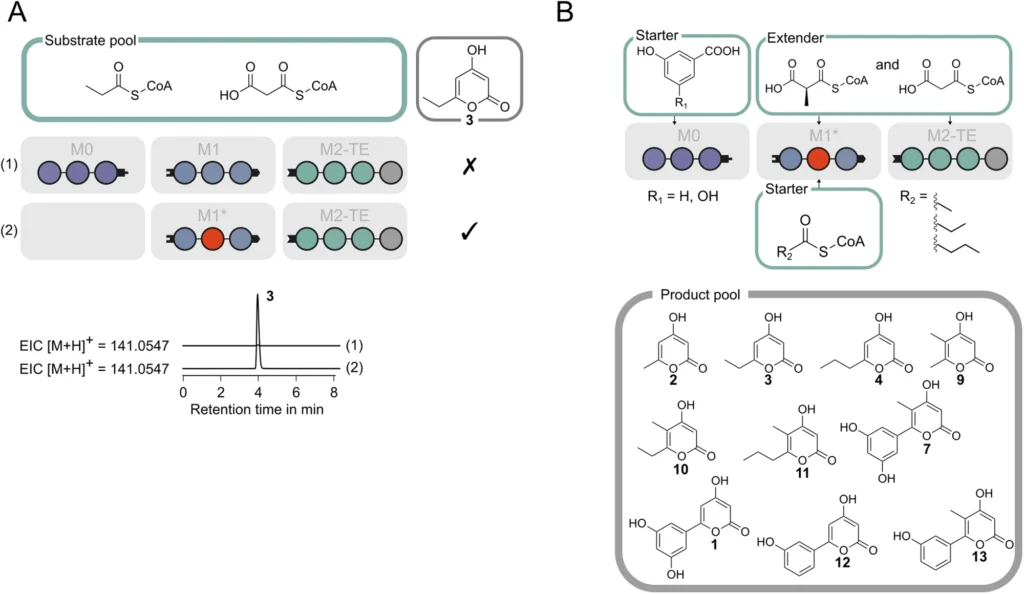
Utilizing MAT to Construct Polyketide Frameworks:
MAT plays a crucial role in the biosynthesis of polyketides:
MAT is an important enzyme in the biosynthesis of polyketides because it helps add different acyl groups to the growing polyketide chain. The capacity to vary the chemical composition of polyketides renders MAT a crucial instrument for creating new molecules with specific biological properties.
Utilizing MAT as a means of expanding the variety of polyketide structures:
MAT’s versatility allows it to readily accept and transfer diverse acyl groups, thereby facilitating modifications to the polyketide backbone. Researchers can increase the chemical diversity of essential natural products by incorporating non-natural substrates into the MAT’s active site, resulting in the production of polyketides with novel and distinct structures.
Case Studies: Effective MAT-facilitated Polyketide Alterations:
Multiple studies have demonstrated the efficacy of MAT in modifying polyketide frameworks. Scientists changed MAT to include fluorinated acyl groups. This made it possible to make fluorinated polyketides that are better at what they do in the body. These case studies demonstrate MAT’s adaptability in polyketide engineering.
The Influence of Multiple Activity Targets (MAT) on a Variety of Scaffold Structures:
The promiscuity of MAT is both advantageous and problematic. On one hand, it facilitates the production of a wide range of polyketide structures. On the other hand, it presents difficulties in regulating the desired result. However, meticulous engineering can utilize this promiscuity to generate a diverse range of innovative molecules with potential therapeutic uses.
MAT’s potential in advancing novel polyketide drug development:
The use of MAT allows for the manipulation of polyketide frameworks, creating novel opportunities for drug discovery. Through the synthesis of distinct polyketides, scientists can produce novel medications that specifically address diseases in ways that current treatments are unable to, perhaps resulting in revolutionary therapeutics. Analysis of AT/MAT-swapped PIKS M5-TE in chemoenzymatic synthesis.
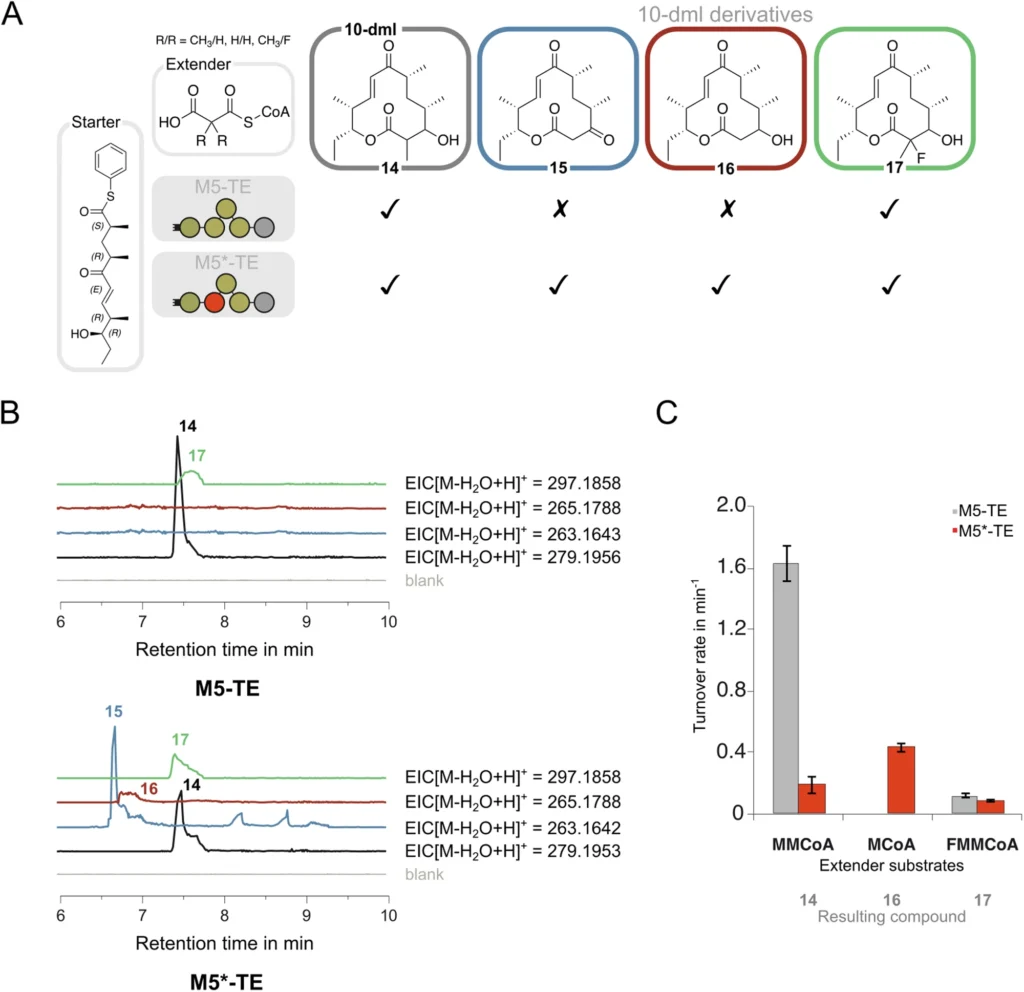
The Benefits of Using Murine MAT in Engineering:
Excessive promiscuity and adaptability:
Murine MAT’s exceptional promiscuity and versatility in accommodating various substrates make it highly esteemed. This to its versatility in introducing various alterations into polyketide structures, this makes it highly suitable for engineering applications.
We conducted a comparative analysis with MATs derived from other species:
Researchers have studied MAT enzymes from other species for polyketide engineering, but murine MAT stands out due to its extensively characterized structure and exceptional efficiency. Comparing murine MAT to MATs from other organisms has shown that it often performs better in terms of its ability to use different substrates and its speed at speeding up reactions.
Enhancing Efficiency in Engineering Elaborate Scaffold Construction:
Murine MAT’s ability to transfer various acyl groups makes it particularly useful for modifying intricate polyketide frameworks. This high level of efficiency enables the quick creation of new compounds, which speeds up the process of discovering and developing drugs.
Integration with Additional Biosynthetic Tools:
The integration of murine MAT with other biosynthetic tools, such as modified PKS enzymes, enables the creation of a comprehensive platform for polyketide engineering. This integration enables the methodical alteration of polyketide structures, resulting in molecules that possess improved or new biological functions.
Constraints and obstacles:
Although murine MAT offers benefits, its application in polyketide engineering presents certain difficulties. The enzyme’s promiscuity, although advantageous in certain situations, can also result in the generation of undesirable byproducts. Furthermore, the need for accurate regulation of MAT activity necessitates the use of advanced technical solutions.
Biotechnology and medical applications:
Customizing Polyketides for Targeted Pharmaceutical Uses:
The use of MAT enables the manipulation of polyketide structures, thereby facilitating the customization of these compounds to meet specific medicinal requirements. By including specific functional groups, scientists can augment the effectiveness of a medication or mitigate its adverse reactions, rendering it more appropriate for therapeutic purposes.
Researching novel antibiotics and anticancer agents:
MAT-mediated polyketide engineering has great potential for creating novel antibiotics and anticancer medicines. Scientists can overcome the limitations of current medications and tackle the escalating issue of antibiotic resistance and cancer drug resistance by synthesizing polyketides with innovative configurations.
The production of agrochemicals based on polyketides presents opportunities:
In addition to pharmaceuticals, MAT-engineered polyketides have potential uses in agriculture. For instance, we could formulate polyketides with insecticidal or herbicidal characteristics as eco-friendly alternatives to conventional agrochemicals.
Factors to Consider in Large-Scale Production:
Industrial applications necessitate a careful assessment of the scalability of MAT-mediated polyketide synthesis. Although laboratory-scale production has achieved success, there are still obstacles to overcome to scale up these processes to meet the expectations of the commercial industry. We anticipate that advancements in fermentation technology and metabolic engineering will significantly contribute to addressing these challenges.
Potential Future Applications of MAT-Engineered Polyketides:
Research is currently underway to expand the range of acyl groups that polyketide scaffolds can incorporate, suggesting a promising future for MAT-engineered polyketides. As our understanding of MAT and its interactions with various substrates becomes more profound, novel prospects for drug discovery and biotechnological applications will continue to arise. Production of 14-membered macrolactones derivatized at C-2 or C-4.
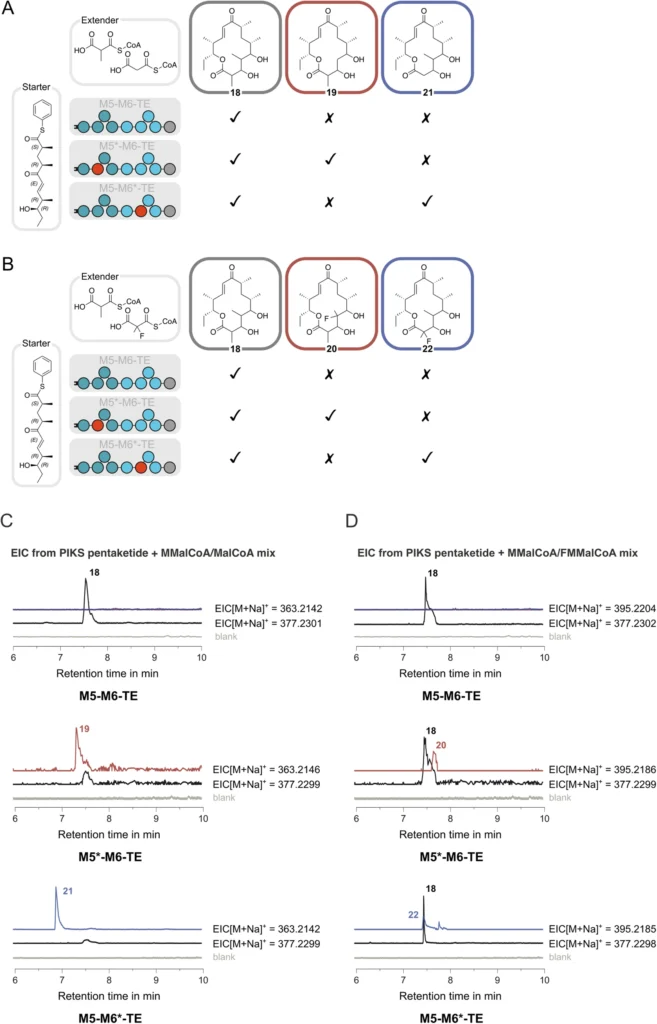
Analysis of Present Studies and Prospective Avenues for Investigation:
The study delves deeper into the application of MAT in the creation of polyketides:
Researchers are currently focussing on understanding the structure that makes MAT promiscuous and making it better at speeding up reactions by using directed evolution and rational design. These endeavors aim to expand the utility of MAT in the domains of synthetic biology and metabolic engineering.
There have been advancements in the field of enzyme engineering:
Advancements in enzyme engineering have led to the creation of MAT variants that exhibit improved specificity and activity. Researchers have altered the active site of MAT to produce enzymes capable of accepting and transferring non-natural substrates. This development expands the potential for polyketide synthesis.
Potential for Synthetic Biology Approaches:
Researchers are investigating the use of synthetic biology techniques to incorporate MAT into artificial biosynthetic pathways. The goal of these endeavors is to develop entirely artificial polyketide-generating systems capable of creating new chemicals on a large scale in industrial settings.
Difficulties in Expanding MAT Applications:
Expanding the use of MAT in industrial applications remains a significant challenge. To fully use the potential of MAT in making a lot of polyketides, problems need to be solved with enzyme stability, substrate supply, and process optimization.
Prospects for long-term growth in the field of drug development:
MAT has a lot of potential in pharmaceutical research because it can change the structures of polyketides in a lot of different ways. This can lead to the discovery of new medicines that work in different ways. We expect magnetic resonance imaging (MRI)-assisted tomography (MAT) to gain more significance as a valuable instrument in the pharmaceutical sector as further investigation in this field progresses.
In conclusion:
The malonyl/acetyl-transferase (MAT) enzyme, which comes from murine fatty acid synthase, is becoming known as a powerful tool for changing polyketide structures because it can work with a wide range of substrates and is flexible. Researchers can use MAT’s ability to incorporate various acyl groups into polyketide structures to generate innovative molecules with significant promise in biotechnology and medicine. Scientists anticipate that the use of MAT (methyltransferase) in polyketide engineering will significantly influence the development of novel pharmaceuticals and other biologically active substances, resulting in significant implications for both health and industry.
Frequently Asked Questions:
1). What is the role of MAT in Fatty Acid Synthase?
MAT makes it easier for malonyl and acetyl groups to move to the acyl carrier protein (ACP). This is a very important step in the process of making fatty acids longer.
2). What role does MAT play in increasing the diversity of polyketides?
The promiscuity of MAT allows it to transfer a variety of acyl groups, facilitating the formation of diverse polyketide structures with distinct biological functions.
3). Why is murine MAT considered a promiscuous enzyme?
The ability of murine MAT to accept and transfer a wide variety of acyl groups beyond its natural substrates qualifies it as promiscuous. This feature makes it extremely adaptable for engineering purposes.
4). What difficulties arise when utilizing MAT for polyketide engineering?
The challenges include regulating the enzyme’s promiscuity to prevent the formation of unwanted byproducts, as well as expanding the process for industrial use.
5). What is the influence of MAT-engineered polyketides on medication development?
MAT-engineered polyketides have the potential to aid in the development of novel medicines with unique structures and modes of action, potentially addressing unmet medical needs.
For more chemistry blogs, visit chemistry Master