Table of Contents
Monoclonal Antibodies and Glycosylation Analysis: An Overview
Monoclonal antibodies (mAbs) have emerged as a potent category of treatments, particularly in oncology, autoimmune disorders, and infectious diseases. Precision medicine relies on these specialized proteins, designed to identify and attach to a specific target antigen. Glycosylation is a critical element that influences monoclonal antibodies’ activity, effectiveness, and overall therapeutic value. Glycosylation denotes the attachment of carbohydrate chains, or glycans, to proteins at designated places. Glycans typically affix to the Fc region of monoclonal antibodies, significantly influencing the antibody’s stability, half-life, and interactions with the immune system.
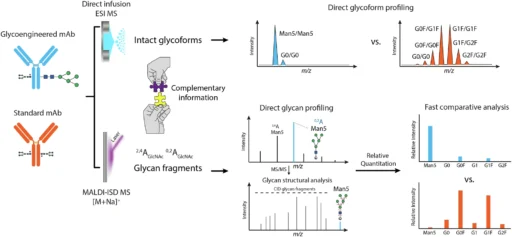
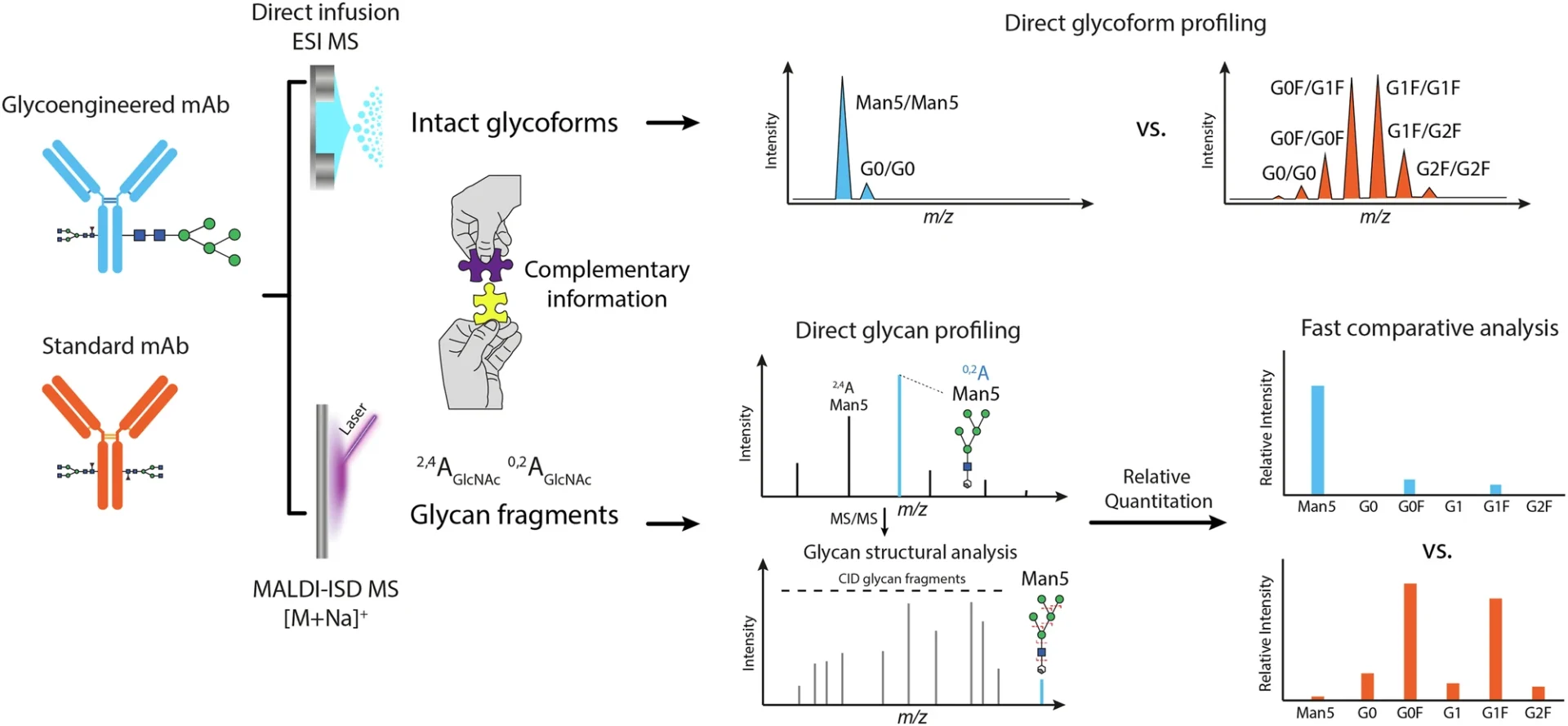
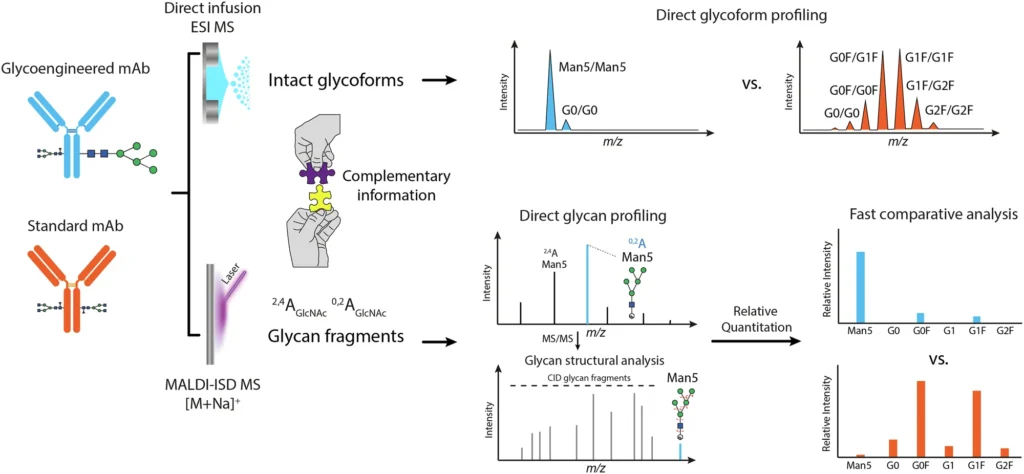
Glycosylation is a complex and heterogeneous process. Rather, it is markedly diverse, indicating that numerous glycoforms—variations of glycan structures—may be present on a single protein. The structure and composition of these glycans are essential since they can affect a monoclonal antibody’s capacity to provoke an immune response, its pharmacokinetics, and its overall therapeutic efficacy. As a result, understanding and accurately evaluating glycosylation is critical in the formulation and manufacture of monoclonal antibodies, particularly for therapeutic applications. Workflow for direct glycosylation analysis from intact standard and glycoengineered mAbs by ESI MS and MALDI-ISD MS.
Obstacles in Glycosylation Analysis:
The complexities of Glycosylation Analysis pose a significant challenge for both scientists and pharmaceutical companies. In contrast to small-molecule medications, monoclonal antibodies are substantial, intricate proteins characterized by diverse glycan structures. Besides their sugar composition, these glycans can vary in branching configurations and connections. Given that these variations may have a significant impact on an antibody’s biological function, it is critical to meticulously analyze each monoclonal antibody’s glycosylation.
An additional problem is that Glycosylation Analysis may fluctuate between production batches during the manufacturing process. Living cells, often Chinese Hamster Ovary (CHO) cells, synthesize monoclonal antibodies (mAbs) as biological medications, contributing to the inherent diversity in the glycosylation process. Even slight variations in glycan structures might influence the efficacy of a monoclonal antibody, rendering it less effective or, in certain instances, provoking adverse immunological reactions. So, accurate and consistent glycosylation analysis is needed to make sure that therapeutic monoclonal antibodies are safe, effective, and follow the rules set by regulators.
Glycosylation Assessment: Conventional Approaches
Traditionally, multi-step procedures have performed glycosylation analysis by enzymatically releasing glycans from the antibody before their examination. A common method is to break down the monoclonal antibody (mAb) with enzymes like PNGase F, which takes out the N-glycan from the protein backbone. Mass spectrometry (MS) and liquid chromatography (LC) generally examine glycans upon their release. These approaches provide a comprehensive structural characterization of glycans, encompassing details regarding sugar kinds, connections, and branching configurations.
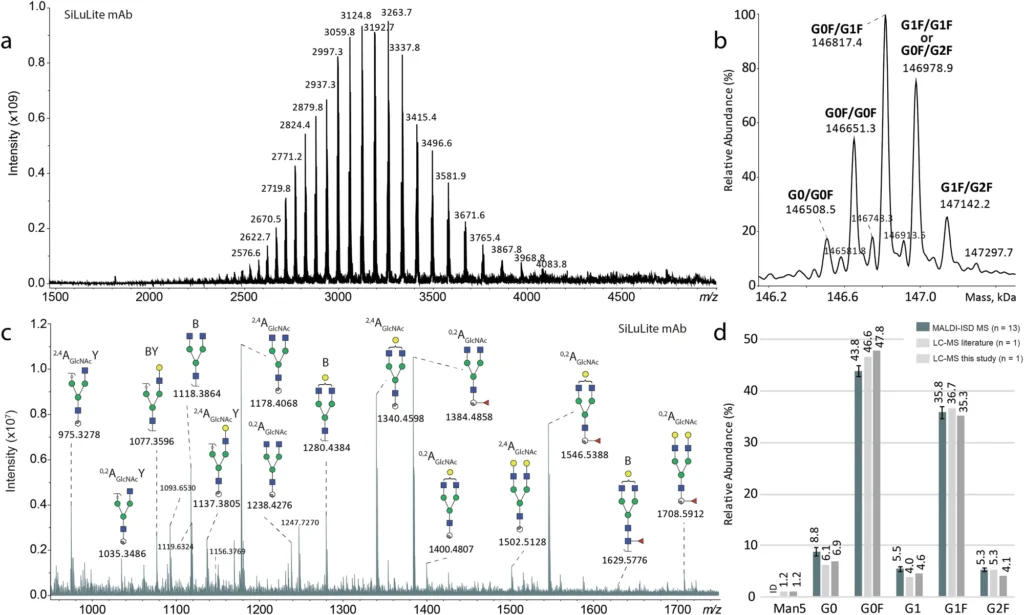
Nonetheless, these conventional approaches possess numerous drawbacks. Initially, they necessitate comprehensive sample preparation, encompassing glycan release, tagging, and purification, which presents the possibility of mistakes and artifacts. Furthermore, these approaches fail to yield information regarding the entire glycoprotein, potentially overlooking significant interactions between the glycan and the protein. So, these methods are good for fully characterizing the structure of glycans, but they don’t give us a full picture of how glycosylation affects the monoclonal antibody (mAb) when it is whole. Direct glycosylation analysis from intact SiLu™Lite MSQC4 mAb expressed in CHO cells.
Direct Glycosylation Analysis: A Transformative Approach
Researchers have come up with new ways to do direct glycosylation analysis of intact monoclonal antibodies, which gets around the problems with the old ways of doing things. This signifies a significant transformation in the discipline, as it allows researchers to examine glycosylation while preserving the natural conformation of the monoclonal antibody. Direct glycosylation analysis preserves the complete protein and its accompanying glycans, providing a comprehensive perspective on the glycosylation landscape.
Direct glycosylation analysis is essential for therapeutic monoclonal antibodies, as preserving the glycoprotein’s integrity is critical for understanding its biological action. Investigating the intact monoclonal antibody enables researchers to understand the influence of glycosylation on the protein’s structure, function, and interactions with other biomolecules. This is especially important in the development of medications, as even minor changes in Glycosylation Analysis can lead to significant differences in clinical outcomes.
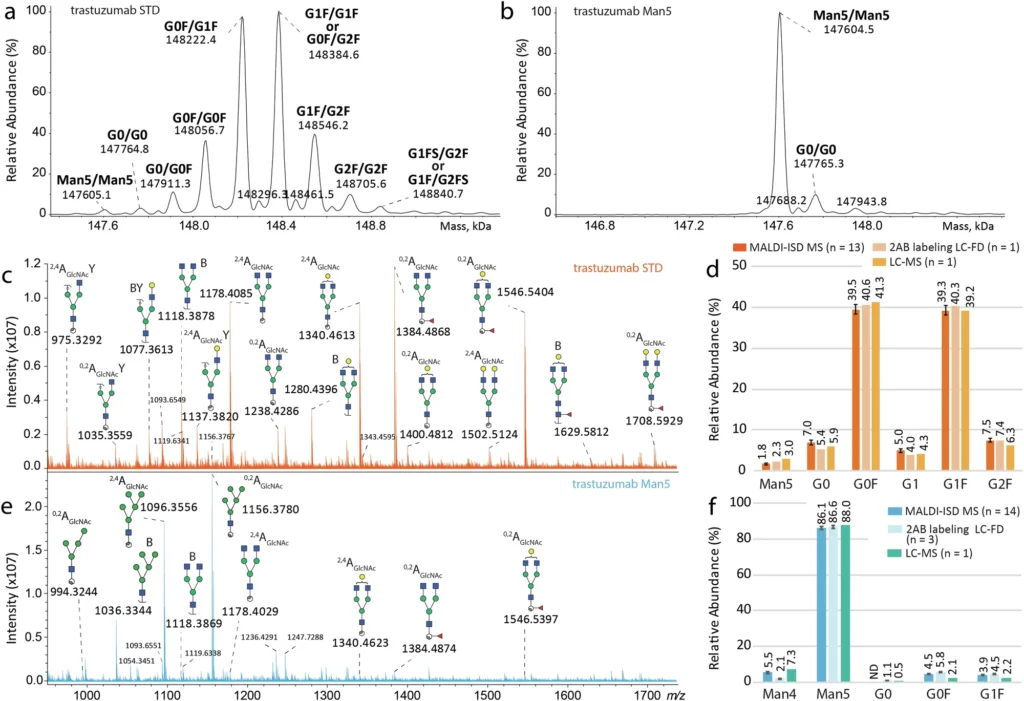
Electric Spray Ionization Mass Spectrometry (ESI MS) and Matrix-Assisted Laser Desorption/Ionization In-Source Decay Mass Spectrometry (MALDI-ISD MS) working together is a very promising way to do direct glycosylation analysis. Collectively, these approaches provide complementary insights into the glycoforms present on the monoclonal antibody as well as the architecture of the glycans themselves. Direct glycosylation analysis from intact standard and glycoengineered trastuzumab mAbs expressed in CHO cells.
Comprehending ESI MS of Glycoforms:
A powerful analytical technique called Electrospray Ionization Mass Spectrometry (ESI MS) has become an important part of protein analysis, especially when looking at large biomolecules like monoclonal antibodies. Delivering a high voltage to a liquid ionizes the sample in ESI MS, creating a fine mist of charged droplets. After the solvent evaporates, the mass spectrometer receives the charged molecules, such as proteins or glycoforms, and assesses their mass-to-charge ratio (m/z).
ESI MS can find and measure the different glycoforms that are on a whole monoclonal antibody for glycosylation analysis. Glycoforms are distinct variations of a protein that arise from the incorporation of diverse glycan structures. ESI MS can profile monoclonal antibody glycoforms by evaluating the entire glycoprotein, eliminating the need for glycan release or substantial sample preparation. ESI MS is an ideal instrument for direct glycosylation investigation because it maintains the mAb’s native conformation and associated glycans.
Glycoform Profiling using Electrospray Ionization Mass Spectrometry:
One great thing about ESI MS for studying glycosylation is that it can give full profiles of all the glycoforms connected to a monoclonal antibody. ESI MS can differentiate between distinct glycoforms by measuring the mass of the intact mAb, which reflects their molecular weight. This allows researchers to determine the relative abundance of each glycoform and detect variations in glycosylation patterns that may influence the monoclonal antibody’s therapeutic capabilities.
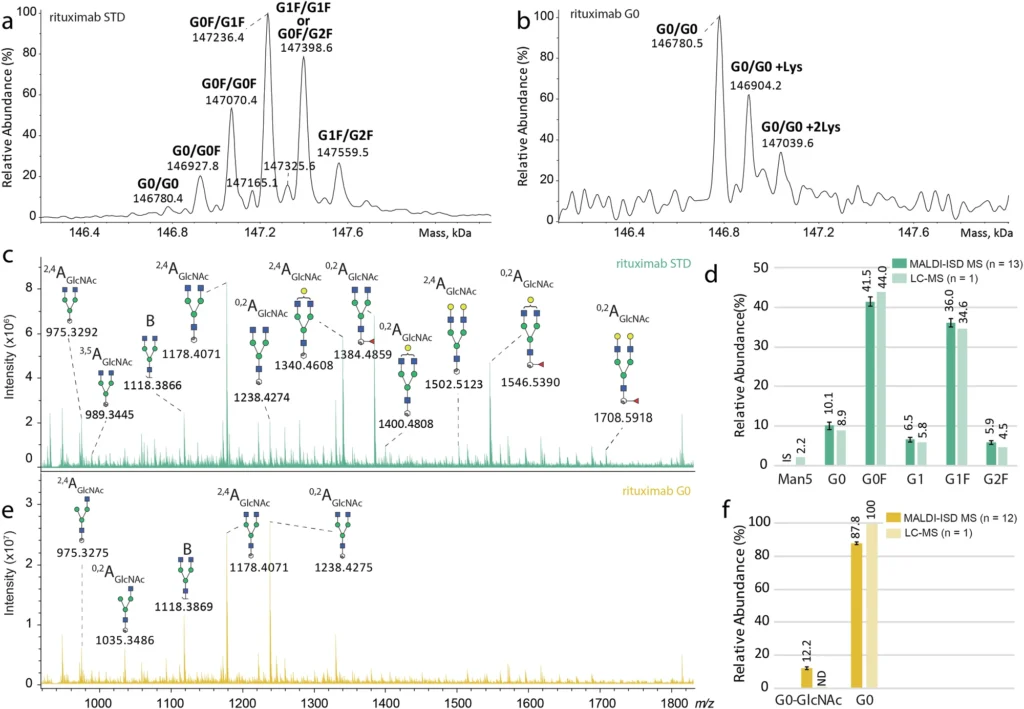
ESI MS can do more than just describe the glycoforms; it can also give information about how the monoclonal antibody’s glycosylation is generally not uniform. Glycosylation is intrinsically diverse, indicating that numerous glycoforms frequently coexist on a single protein. Researchers the analysis of mass spectra derived from ESI MS, researchers may quantify this variability and evaluate its potential effects on the antibody’s functionality. Consistent glycosylation is crucial in the development of therapeutic monoclonal antibodies to guarantee the drug’s safety and efficacy. Direct glycosylation analysis from intact standard and glycoengineered rituximab mAbs.
Introduction to MALDI-In Source Decay Mass Spectrometry:
Although ESI MS is a proficient instrument for profiling intact glycoforms, it fails to deliver comprehensive insights into the structural composition of the glycans. Researchers frequently utilize Matrix-Assisted Laser Desorption/Ionization (MALDI), a potent mass spectrometry technique. MALDI works by ionizing the material with a laser, allowing for the analysis of large macromolecules with minimal fragmentation. This is particularly advantageous for the examination of proteins, peptides, and glycans.
MALDI is particularly useful in glycosylation analysis for in-source decay (ISD) mass spectrometry. ISD is a type of fragmentation that transpires within the ionization source, generating pieces of the examined molecule. MALDI-ISD MS can fragment glycans linked to a monoclonal antibody in glycosylation, yielding comprehensive structural information.
The Mechanism of MALDI-ISD Mass Spectrometry:
The laser ionizes the glycans attached to the monoclonal antibody in MALDI-ISD MS, fragmenting them. This generates a collection of smaller fragments that can be examined to determine the glycos’ composition and structure. These pieces may consist of individual monosaccharides (e.g., glucose, mannose, or fucose) and more intricate structures, such as branching oligosaccharides. Researchers can infer critical structural information about glycans by analyzing fragmentation patterns, including the types of sugars present, their connections, and their overall structure.
The capacity to fragment glycans and analyze their structure is crucial in the realm of therapeutic monoclonal antibodies, as the glycan configuration might affect the antibody’s biological function. Some sugar residues, like fucose, can change how well a monoclonal antibody works to cause antibody-dependent cellular cytotoxicity (ADCC), which is an important way that many cancer treatments work.
Integration of ESI MS and MALDI-ISD MS for Glycosylation Examination:
The integration of ESI MS and MALDI-ISD MS underpins the efficacy of contemporary glycosylation analysis. ESI MS gives a detailed picture of the glycoforms connected to the whole mAb, while MALDI-ISD MS gives very detailed information about the structures of the glycans. Researchers can get a full picture of the glycosylation landscape by using both of these methods at the same time. This includes both the general glycoform profile and the finer details of glycan structure.
Collaborative Insights from ESI MS and MALDI-ISD MS:
The synergistic characteristics of ESI MS and MALDI-ISD MS make them a formidable duo for glycosylation research. ESI MS is very good at identifying intact glycoproteins and giving information about the amount and frequency of different glycoforms. This is critical for understanding comprehensive glycosylation heterogeneity and ensuring batch-to-batch consistency in monoclonal antibody manufacturing.
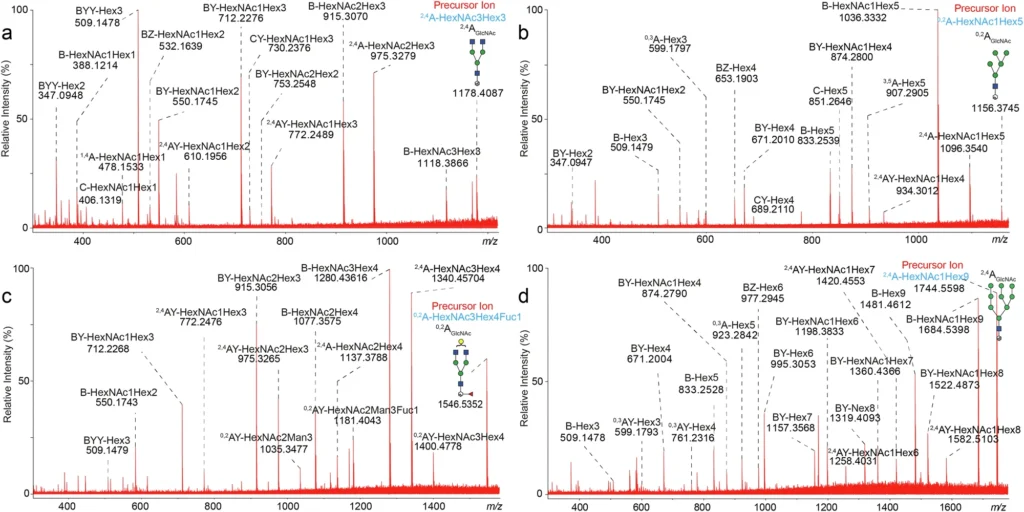
The MALDI-ISD MS technique gives researchers a lot of information about glycan structure, like how it branches, how much monosaccharide it contains, and how the chains connect. By using both of these methods together, researchers can see glycosylation from both a large (ESI MS) and a small (MALDI-ISD MS) angle. This gives them a better idea of how glycosylation affects the functionality of mAbs. MALDI-ISD CID FT-ICR mass spectra of glycan fragment ions.
How MALDI-ISD MS Elucidates Glycan Structures:
A significant advantage of MALDI-ISD MS is its capacity to fragment glycans in a controlled and predictable fashion. We can examine the fragments generated during ISD to understand the glycan’s structure, including its branching configurations and the composition of distinct sugar residues. This information is critical for understanding how glycosylation affects the biological activity of monoclonal antibodies.
Researchers recognize that glycans with fucose residues diminish the efficacy of monoclonal antibodies in mediating antibody-dependent cellular cytotoxicity (ADCC), a crucial mechanism in numerous cancer therapies. By using MALDI-ISD MS to look at the structure of glycans connected to a monoclonal antibody, researchers can find fucose and pinpoint exactly where it is. This information is essential for enhancing the therapeutic efficacy of the monoclonal antibody.
Benefits of the Integrated ESI MS and MALDI-ISD Methodology:
The integration of ESI MS and MALDI-ISD MS provides numerous significant benefits for glycosylation analysis. Firstly, it makes it easier to look at intact monoclonal antibodies directly, without having to do a lot of sample processing or glycan release. This keeps the monoclonal antibody’s native shape and the glycans that are attached to it, giving a clearer and more complete picture of the glycosylation landscape.
This integrated methodology provides superior resolution and precision relative to conventional techniques. ESI MS gives complete profiles of whole glycoproteins, while MALDI-ISD MS gives detailed structural information about the glycans. Altogether, these methods give us a full picture of how glycosylation affects the monoclonal antibody, including both the broad profile of glycoforms and the finer details of glycan structure.
The integrated ESI MS and MALDI-ISD methodology is exceptionally effective, allowing for rapid examination of many glycoforms and glycans in a single experiment. Rapid and precise glycosylation screening is crucial in the pharmaceutical sector to guarantee that therapeutic monoclonal antibodies comply with regulatory norms.
Practical applications in biopharmaceutical development:
The biopharmaceutical sector extensively uses the integrated ESI MS and MALDI-ISD MS methodologies for the advancement and manufacturing of monoclonal antibodies. A crucial use of this method is to guarantee that therapeutic monoclonal antibodies exhibit uniform glycosylation profiles across several production batches. This is essential for preserving the drug’s efficacy and safety, as minor alterations in glycosylation can substantially impact the therapeutic qualities of the antibody.
Cancer medicines that use ADCC as a mechanism of action commonly employ glycosylation analysis. Researchers can improve the monoclonal antibody’s ability to help antibody-dependent cellular cytotoxicity (ADCC) and make it more effective at killing cancer cells by making sure it has the right glycosylation pattern, such as reduced fucosylation.
Optimizing Quality Control in Monoclonal Antibody Production:
This integrated method is particularly useful in quality assurance during the production of monoclonal antibodies. ESI MS and MALDI-ISD MS are quick and accurate ways to test for glycosylation, which lets manufacturers make sure that every batch of mAbs meets the necessary glycosylation standards. This is crucial for regulatory adherence, especially with agencies such as the U.S. The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) mandate comprehensive analysis of glycosylation for biologic pharmaceuticals.
Besides guaranteeing regulatory compliance, glycosylation analysis is crucial for maintaining batch-to-batch consistency in monoclonal antibody production. By examining each batch’s glycoform profile and glycan structures, manufacturers can identify any discrepancies in glycosylation that may influence the monoclonal antibody’s therapeutic qualities. This guarantees that patients receive consistent, uniform, and effective treatment.
Possible Constraints of Direct Glycosylation Assessment:
The integrated ESI MS and MALDI-ISD MS methodology presents numerous benefits, although it also possesses certain drawbacks. The technical complexity of the methodologies involved is a primary problem. Both ESI MS and MALDI-ISD MS necessitate specialized apparatus and knowledge, rendering them less accessible to laboratories without the requisite resources.
The expense associated with these methodologies is another constraint. The apparatus necessary for ESI MS and MALDI-ISD MS is costly, and the analysis can be protracted and laborious. These approaches’ sensitivity may be insufficient for detecting low-abundance glycans, making it difficult to study monoclonal antibodies with minimal glycosylation.
Actually, the combined ESI MS and MALDI-ISD MS method gives a lot of information about glycosylation, but it might not work with all types of monoclonal antibodies or glycan structures. Some glycans may exhibit excessive complexity or heterogeneity, rendering them inadequately described by current techniques and necessitating additional methodologies or technologies for comprehensive investigation.
Prospective Avenues for Glycosylation Analysis:
We expect advancements in mass spectrometry technology and the incorporation of artificial intelligence (AI) for data processing to influence the future of glycosylation analysis. An important trend is the development of more sensitive and high-resolution mass spectrometry instruments, which enable the detection and characterization of complex and low-abundance glycans.
Another notable advancement is the use of AI and machine learning to automate mass spectrometry data examination. Right now, it takes a lot of skill to figure out what mass spectrometry data means. But artificial intelligence could make data processing faster by automatically recognizing glycoforms, glycan structures, and fragmentation patterns. This may expedite glycosylation analysis, enhance its accuracy, and broaden accessibility for a greater number of laboratories.
As mass spectrometry (MS) technology improves, scientists are looking into new ways to analyze glycosylation directly that go beyond electrospray ionization mass spectrometry (ESI MS) and matrix-assisted laser desorption/ionization in-source decay mass spectrometry (MALDI-ISD MS). Researchers are examining techniques like ion mobility spectrometry (IMS) and top-down proteomics to gain more comprehensive insights into glycosylation at the molecular level.
Summary:
The direct glycosylation analysis of whole monoclonal antibodies using ESI MS and MALDI-ISD MS is a big step forward in the development of biopharmaceuticals. This method surpasses existing techniques by maintaining the native structure of the mAb and its associated glycans, offering a more precise and thorough understanding of glycosylation. It has the twin advantage of characterizing entire glycoforms while disclosing intricate structural details regarding the glycans.
With the increasing use of therapeutic monoclonal antibodies, precise analysis and regulation of glycosylation will be critical for ensuring the safety, efficiency, and consistency of these essential medications. The integration of ESI MS and MALDI-ISD MS methodologies is currently pivotal in medication development, production, and quality assurance, and its significance is anticipated to increase in the coming years.
Frequently Asked Questions:
1). What is glycosylation, and what is its significance in monoclonal antibodies (mAbs)?
Glycosylation is the conjugation of carbohydrate molecules (glycans) to proteins, such as monoclonal antibodies (mAbs). It influences the stability, functionality, and interactions of the antibody with the immune system, rendering it essential for therapeutic efficacy.
2). What is the role of ESI MS in glycosylation analysis?
ESI MS describes the different glycoforms (multiple glycan structures) that are found on the whole mAb. This gives detailed information about the protein’s mass and glycosylation while keeping its shape.
3). What significance does MALDI-ISD MS have in glycosylation analysis?
MALDI-ISD MS breaks the glycans linked to monoclonal antibodies, allowing researchers to examine their structure, including branching and sugar content, which are critical for understanding the mAbs’ therapeutic efficacy.
4). What are the advantages of combining ESI MS and MALDI-ISD MS for glycosylation analysis?
The integration yields a comprehensive perspective: ESI MS shows glycoforms that are still whole, while MALDI-ISD MS lets you look at complex glycan structures, which lets you fully understand how glycosylation affects the functionality of mAbs.
5). What are the difficulties associated with using ESI MS and MALDI-ISD MS for glycosylation analysis?
The primary hurdles include the equipment’s high costs and technical intricacies, labor-intensive analytical procedures, and possible constraints in identifying low-abundance glycos.
For more chemistry blogs, visit chemistry Master