Table of Contents
The opening statement of H2 Roaming Reactions:
In modern chemistry, hydrogen (H2) is an essential element. Many different chemical reactions and industrial processes consider hydrogen (H2) as a fundamental component due to its widespread presence and versatility. The concept of wandering reactions is one of the fascinating phenomena related to hydrogen. These reactions, which exhibit non-traditional routes and intermediate steps, present a challenge to standard chemical reaction theories and provide fresh perspectives on molecular dynamics. This paper examines the real-time monitoring of H2 roaming reactions, focusing on the technological progress, experimental configurations, difficulties, and prospects in this captivating area of research.
Analyzing H2 Roaming Reactions:
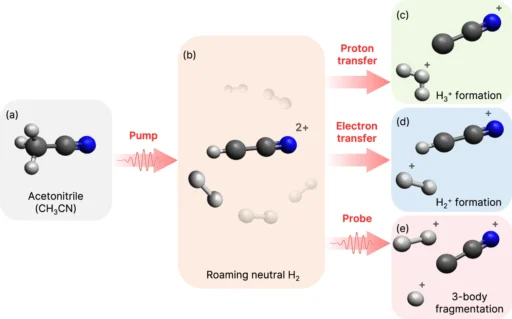
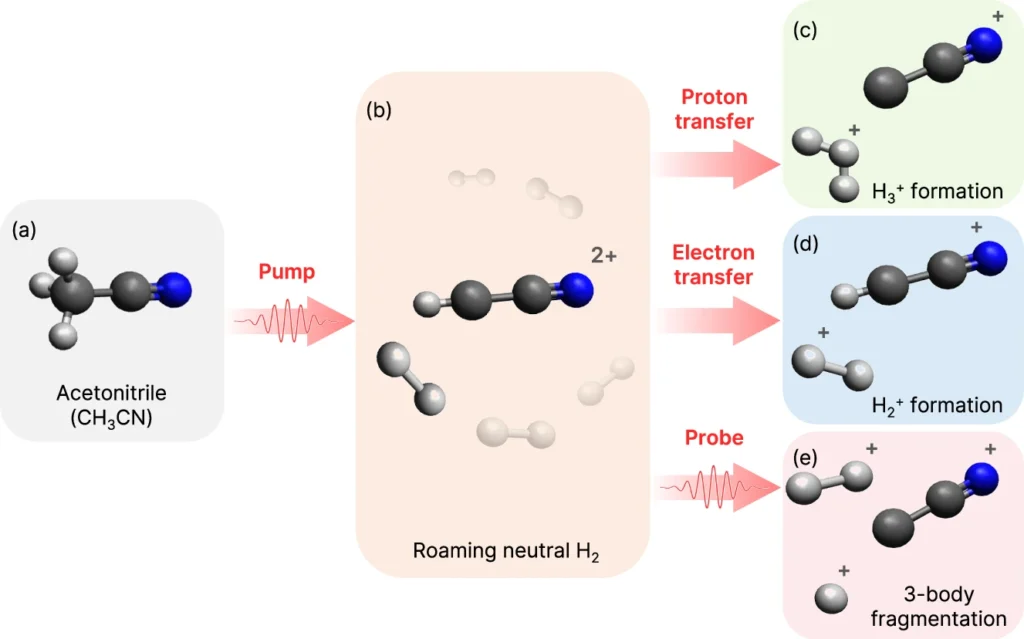
Researchers first observed roaming reactions, a relatively discovery in chemistry, in the early 2000s while studying the breakdown of formaldehyde. H2 Roaming reactions, in contrast to conventional reactions, entail the fragmentation of a molecule, followed by the free movement of its fragments, before ultimately generating products. Potential energy surfaces (PES) observe this wandering tendency, where the fragments move around flat areas before finding a pathway to escape and form products. This notion has greatly transformed our comprehension of molecular dynamics and reaction processes. Schematic representation of H2 roaming induced by the pump pulse and the resultant product channels in CH3CN (N atom in blue, C atoms in dark gray, and H atoms in light gray).
The Scientific Explanation of Roaming Reactions:
The concept of potential energy surfaces (PES) is fundamental to understanding wandering reactions. Potential Energy Surfaces (PES) depict the configuration of energy levels that molecules navigate while undergoing a chemical reaction. During a wandering reaction, a molecule’s dissociation does not result in quick recombination or the formation of new chemical bonds. Instead, they meander throughout the Potential Energy Surface (PES), investigating several arrangements before ultimately adopting a stable result. Small differences in the potential energy surface (PES) influence this behavior, leading to the development of unexpected products. Comprehending this necessitates advanced theoretical models and computational simulations that can precisely depict these intricate energy environments.
Techniques for monitoring chemical reactions:
Monitoring chemical reactions has always been difficult because of the fleeting existence of several intermediary substances and the rapid timeframes in which these reactions take place. Conventional methods like mass spectrometry, nuclear magnetic resonance (NMR), and X-ray crystallography have proven quite beneficial in offering insight into reaction mechanisms. Nevertheless, these techniques frequently fail to capture the swift and transitory events that are typical of wandering reactions. The demand for immediate monitoring has spurred the development of more sophisticated methods that can provide comprehensive images of molecular interactions in real-time.
Recent technological advancements have enabled real-time tracking:
In recent years, advancements in spectroscopy and computational chemistry have significantly enhanced our ability to monitor chemical reactions in real-time. Scientific methods like femtosecond laser spectroscopy allow scientists to detect chemical interactions within extremely brief time intervals, as short as a few femtoseconds (10^-15 seconds). This enables the acquisition of the ephemeral states and nomadic behaviors that are essential for comprehending these reactions. Moreover, computational models have progressively advanced, enabling the precise simulation of these reactions. These models can enhance experimental data, offering a more thorough understanding of the kinetics of the reaction.
We are tracking H2 roaming reactions directly:
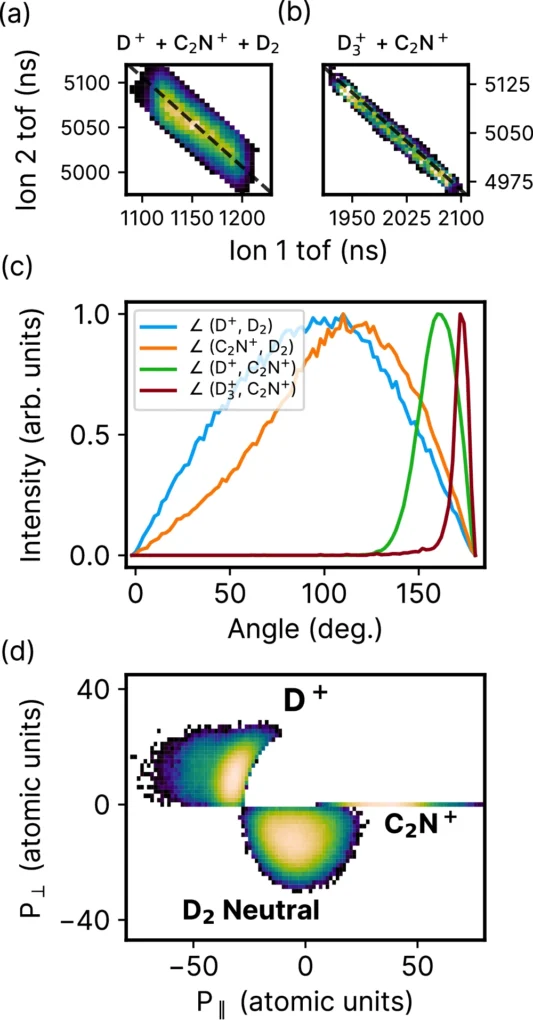
Researchers use a combination of sophisticated experimental settings and real-time monitoring approaches to directly detect H2 roaming reactions. Researchers frequently combine high-resolution spectroscopy with ultrafast lasers to directly observe the dissociation and wandering of hydrogen molecules in real-time. We carefully craft these experiments to capture the brief instances of molecular movement. Pump-probe spectroscopy, for example, is a widely used technique for monitoring these processes. A laser pulse, known as the pump, initially stimulates the molecules in this method, and a second pulse, known as the probe, then measures the effects. We subsequently examine the data from these studies to understand the reaction mechanisms and the characteristics of the roaming behavior.
Live monitoring led to significant discoveries:
By conducting real-time tracking of H2 roaming reactions, researchers have gained valuable and noteworthy information. Scientists have noted that the act of roaming can result in the creation of unforeseen substances, which contradicts conventional ideas about how reactions occur. These findings have significant consequences for our comprehension of response dynamics, indicating that molecules exhibit more intricate and diverse behaviors than previously believed. Studies have demonstrated that, under specific circumstances, hydrogen atoms can travel greater distances before undergoing a reaction, resulting in the creation of new and unique substances. These insights not only improve our theoretical comprehension but also have practical consequences for developing more effective chemical processes and catalysts. Simulation of roaming H2 and the corresponding potential energy surface.
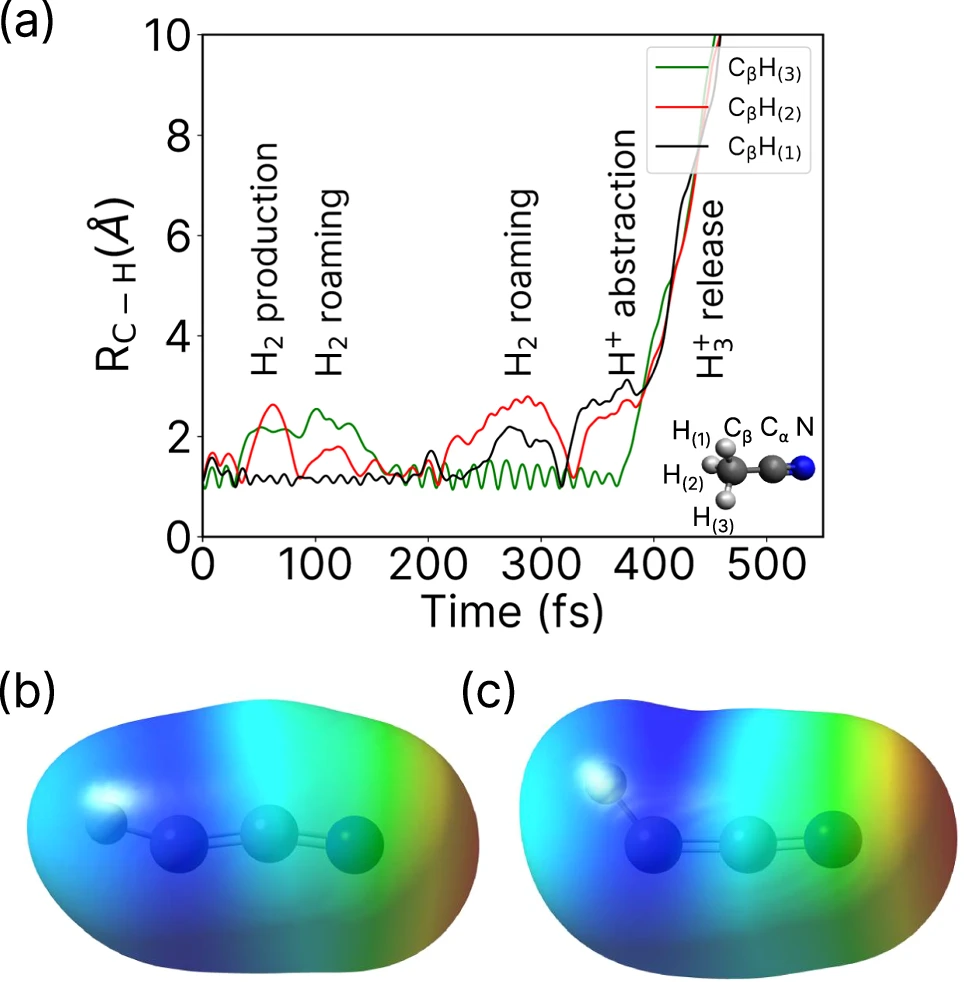
Difficulties in Monitoring H2-Roaming Reactions:
Tracking the movements of H2 roaming reactions in real-time is difficult, despite the progress made in technology. The evanescent nature of these interactions necessitates exceedingly rapid and discerning detection techniques. Femtosecond laser spectroscopy is a very advanced technique that necessitates meticulous calibration and control due to its technical complexity. Moreover, the process of analyzing the vast quantities of data produced by these studies is an intricate undertaking. The H2 Roaming Reactions activity frequently results in a diverse array of potential consequences, which complicates the process of unraveling the exact pathways and mechanisms at play. This requires the utilization of sophisticated computing tools and algorithms to efficiently evaluate and interpret the data.
The study involves the analysis of specific instances and practical implementations:
Multiple notable studies have demonstrated that H2 roaming reactions can be tracked in real time. The initial detection of the wandering phenomenon occurred during the disintegration of formaldehyde (H2CO). In this H2 Roaming reactions, rather than undergoing direct decomposition into carbon monoxide (CO) and hydrogen (H2), the molecule dissociates into H2 and a mobile hydrogen atom.
This hydrogen atom subsequently interacts with the remaining formyl radical (HCO) to produce CO and H2. This result significantly impacts our understanding of combustion processes, given the observation of similar wandering behaviors in other small molecules involved in burning. Practical applications include areas such as atmospheric chemistry and astrochemistry, where understanding hydrogen interactions can result in more precise models of chemical processes in a variety of settings.
There are prospects for advancements in H2 roaming reactions research:
The future of research on H2 Roaming Reactions depends on the incorporation of developing technologies and integrative methodologies. The progress in machine learning and artificial intelligence can efficiently evaluate intricate datasets, revealing previously concealed patterns and trends. For instance, we can train artificial intelligence algorithms to identify specific movement patterns and predict their responses under different conditions. Moreover, the advancement of more rapid and highly responsive detection techniques would significantly improve our capacity to investigate these interactions in real time. Upcoming advancements like attosecond spectroscopy, which measures time intervals of 10^-18 seconds, have the potential to achieve even more precision in studying the temporary states associated with wandering reactions.
The influence on chemical synthesis and catalysis is significant:
Gaining a comprehensive understanding of wandering reactions carries substantial implications for the fields of chemical synthesis and catalysis. Through the disclosure of novel chemical pathways, scientists can develop catalysts that are more effective and specific in their function. This understanding can facilitate the creation of novel synthetic techniques that minimize waste and enhance the sustainability of chemical processes.
H2 Roaming reactions studies can provide valuable insights for the synthesis of medicines, particularly in cases where accuracy and selectivity are of utmost importance. We can design catalysts to minimize by-product production and enhance overall yield by utilizing these insights. Moreover, in industrial applications such as hydrogen manufacturing and fuel cell technology, comprehending the roaming patterns of hydrogen molecules can result in enhanced efficiency and cost-effectiveness.
Partnerships and cross-disciplinary methods:
The investigation of wandering reactions is intrinsically interdisciplinary, necessitating collaboration among chemists, physicists, and computational scientists. These interactions are critical for improving our comprehension and creating innovative technology. Interdisciplinary techniques promote creativity by combining diverse viewpoints and expertise to address intricate scientific difficulties. Collaborations between experimental chemists and computational scientists can result in more precise models and simulations. Similarly, partnerships with engineers can help advance sophisticated detection systems. Combining insights from many disciplines can result in significant advancements that would be challenging to accomplish independently. Time-dependent signal intensities of competing channels.
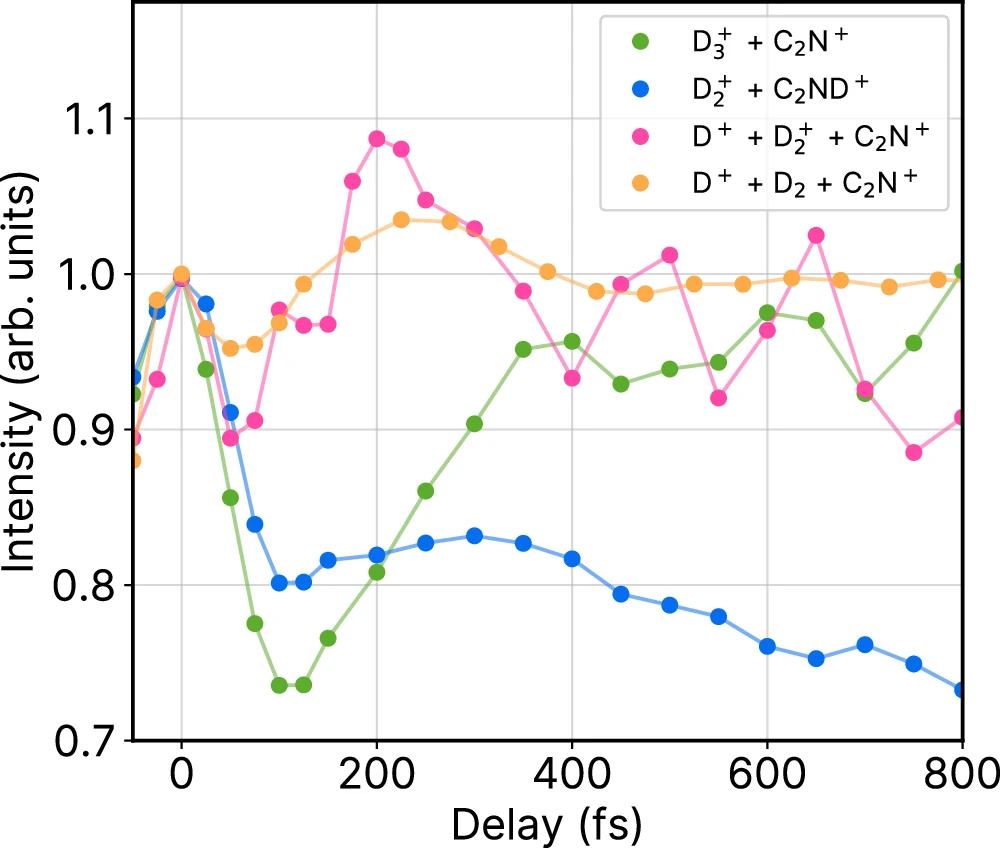
We must consider ethical and environmental factors:
When doing sophisticated scientific research, it is crucial to prioritize ethical and environmental factors. When studying hydrogen processes, it is important to consider their potential environmental consequences. Improper handling of hydrogen, despite being a clean fuel, can lead to environmental consequences. To illustrate, we must effectively control the process of producing and storing hydrogen to prevent leakage and minimize its negative environmental impact. Ethical considerations include the conscientious use of technology and the communication of research outcomes in a manner that is beneficial to society at large. Researchers must prioritize ethically conducting their work, paying close attention to sustainability, and considering the potential consequences for future generations.
In conclusion:
The real-time monitoring of H2 roaming reactions makes a significant contribution to our understanding of chemical dynamics. Researchers are using advanced technologies and transdisciplinary methods to discover novel ways in which reactions occur and question existing hypotheses. The ramifications for chemical synthesis, catalysis, and larger scientific knowledge are significant, indicating the beginning of a new age in chemistry. As our knowledge and technological capabilities progress, the possibilities for innovation in several scientific and industrial domains are vast. The exploration of wandering reactions is in its early stages, and there are promising prospects for future discoveries and applications.
Frequently Asked Questions:
1). A wandering reaction refers to a type of chemical reaction where the reactant molecules undergo a non-linear pathway, leading to the formation of unexpected products.
A wandering reaction is a process in which a molecule breaks apart and its pieces move independently before recombining or producing new products. This deviates from the conventional pathways followed by reactions. The potential energy surfaces that the fragments move across determine the behavior.
2). What is the significance of H2 in chemical reactions?
Hydrogen (H2) plays a crucial role in numerous chemical processes, functioning as a reactant, product, or intermediate in a variety of reactions. Due to its compact dimensions and strong reactivity, it plays a crucial role in various domains, such as energy generation, industrial synthesis, and atmospheric chemistry.
3). What are the primary techniques employed for monitoring chemical reactions?
Conventional techniques comprise mass spectrometry, nuclear magnetic resonance (NMR), and X-ray crystallography. We employ advanced methodologies like femtosecond laser spectroscopy and computational simulations to monitor and analyze the rapid changes and dynamic behaviors occurring in chemical reactions in real time.
4). What challenges arise when monitoring H2 roaming reactions in real-time?
The challenges include the need for very rapid detection techniques to record momentary variables, the complexity of analyzing large datasets, and the technological requirements of maintaining accurate control over experimental circumstances. Efficient data analysis and interpretation typically necessitate the use of advanced computational techniques.
5). What is the potential impact of breakthroughs in this field on the industry?
Technological progress can lead to more efficient catalysts, improved synthesis techniques, and a deeper understanding of reaction mechanics. Enhancing the efficiency and sustainability of chemical processes can be advantageous for multiple industries, such as pharmaceuticals, energy generation, and materials research.
For more chemistry blogs, visit chemistry Master