Table of Contents
Overview:
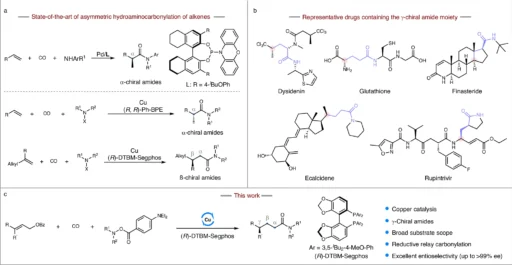
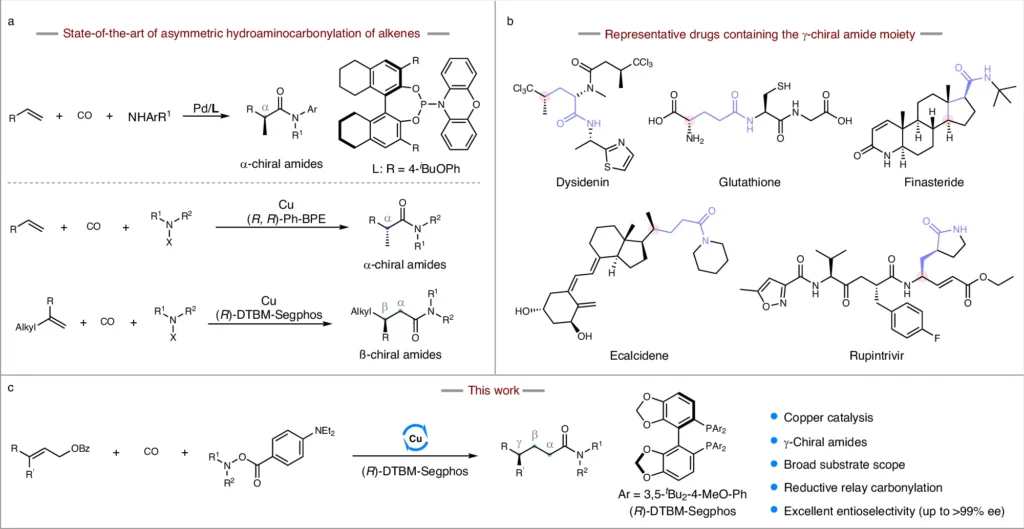
Within the field of pharmaceutical chemistry, the process of creating chiral amides is a crucial undertaking. Chiral amides, especially those containing a γ-chiral core, play a vital role as intermediates in synthesizing many active pharmaceutical ingredients (APIs). We cannot overstate the significance of synthesizing these compounds with enantioselectivity, as it directly influences pharmaceuticals’ effectiveness and safety features. This article details how to make γ-chiral amides using a copper-catalyzed reductive relay hydroaminocarbonylation method for enantioselective synthesis. This novel approach provides increased productivity, greater specificity, and the possibility for extensive utilization in pharmaceutical synthesis.
Context:
To understand the background of this synthesis, one must have a solid understanding of enantioselective synthesis and the significance of γ-chiral amides. In the field of medication research, enantioselective synthesis is a deliberate procedure that aims to generate a particular enantiomer of a chemical. The biological activity of different enantiomers of a chemical can vary significantly.
Therefore, it is crucial to produce the correct enantiomer. γ-Chiral amides, which have a chiral center located at the gamma position to the amide group, are of significant importance. These chemicals play a crucial role in the composition of different medications, affecting their ability to bind to receptors, their metabolism in the body, and their overall effectiveness in treating medical conditions. Catalytic asymmetric hydroamincarbonylation and alkenes to chiral amides.
Catalytic reactions involving copper:
Copper-catalyzed reactions have become a fundamental and essential component in contemporary organic synthesis. There are several reasons for this: Copper is reasonably affordable and widely available, and copper catalysts often demonstrate strong reactivity and selectivity. The unique attributes of copper make it a compelling option for intricate synthetic reactions. Copper catalysts are very important in enantioselective synthesis because they help make chiral centers very accurately, which makes the process more efficient and selective overall.
The hydroaminocarbonylation process:
A very important reaction in organic chemistry is hydroaminocarbonylation, which turns amines into amides by mixing them with alkenes and carbon monoxide (CO). The ability of this reaction to form C-N bonds, commonly found in medicinal molecules, makes it highly significant. You can customize the hydroaminocarbonylation technique to generate a range of amides, making it a flexible instrument in the synthesis of intricate compounds. As a whole, the process involves attaching the alkene and CO to a metal center, then adding the amine, and finally making the amide bond.
Hydroaminocarbonylation via relay:
Relay reactions aim to enhance efficiency and reduce waste during multi-step synthesis by transferring an intermediate through multiple steps without isolation. Implementing a relay strategy in hydroaminocarbonylation can greatly enhance the overall production and specificity. By improving each step in the reaction sequence, relay hydroaminocarbonylation can effectively lower the number of unwanted side reactions and make the whole process easier. This method is especially beneficial when working with delicate intermediates or when striving to optimize the production of desired products.
Reductive Relay Hydroaminocarbonylation:
We achieve an additional level of control and efficiency by including a reductive ingredient in the relay hydroaminocarbonylation process. The reductive relay hydroaminocarbonylation process makes the intermediate compounds more stable and increases the production of γ-chiral amides with a high level of enantioselectivity. In this setting, reductive agents play a crucial role in preserving the desired oxidation state of intermediates, hence facilitating a seamless progression through the reaction process. This technique is particularly efficient in regulating the stereochemistry of the end product, which is essential for synthesizing molecules that are enantiomerically pure.
Enantioselectivity in synthesis:
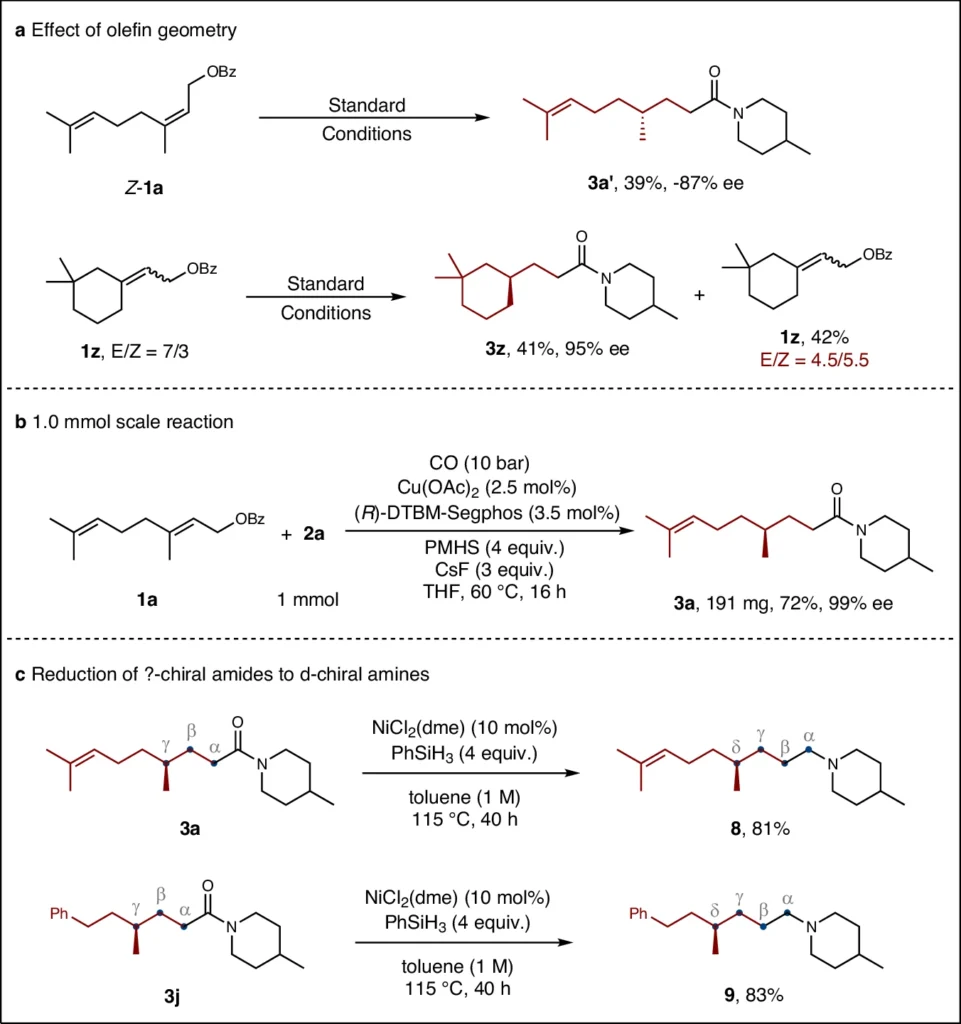
Attaining enantioselectivity is a primary obstacle in organic synthesis. Enantioselectivity occurs when a chemical process forms one enantiomer in greater amounts than the other. Selectivity is critical in the pharmaceutical industry because the intended therapeutic impact of a medication is frequently associated with a specific enantiomer. Different techniques, such as utilizing chiral catalysts, chiral auxiliaries, and chiral surroundings, can achieve enantioselectivity. In copper-catalyzed reductive relay hydroaminocarbonylation, the copper catalyst is very important for controlling the creation of the chosen chiral center. Effect of olefin geometry and synthetic application.
Mechanistic investigations and proposed mechanism.
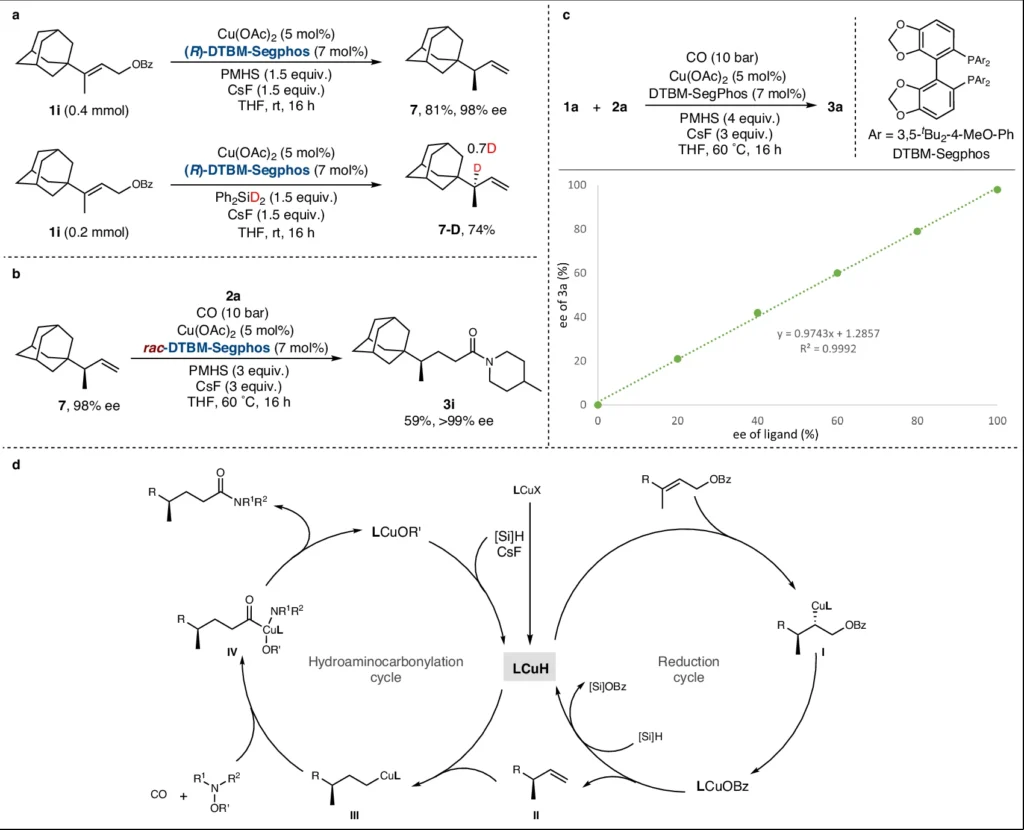
Copper-catalyzed reductive relay hydroaminocarbonylation:
The copper catalyst plays a crucial role in the enantioselective synthesis of γ-chiral amides. The intricate system entails multiple crucial stages:
Activation of the Alkene and CO: The copper catalyst forms coordination bonds with the alkene and carbon monoxide, enabling their activation.
Addition of the Amine: The addition of the amine to the activated complex causes a nucleophilic assault and the formation of an intermediate complex.
Reductive Elimination: A reductive agent facilitates reductive elimination by stabilizing the intermediate and supporting the final step. This leads to the creation of the γ-chiral amide.
The copper catalyst not only aids in these processes but also improves enantioselectivity by creating a chiral environment that promotes the synthesis of one enantiomer over the other.
Experimental parameters:
The best reaction conditions are very important for copper-catalyzed reductive relay hydroaminocarbonylation to work well. Important factors to consider are:
Temperature: People often use moderate temperatures to strike a balance between response speed and selectivity.
Pressure: Maintaining the appropriate levels of CO pressure is crucial for achieving and sustaining reaction equilibrium.
Solvent Choice: The choice of solvent can have a significant impact on a process’s efficiency and selectivity. Preferential solvents are those that stabilize intermediates and improve the interaction between reactants and catalysts.
It is essential to meticulously adjust these conditions to achieve the highest possible yield and enantioselectivity while simultaneously eliminating any unwanted side reactions.
Range of Substrates:
This synthetic approach demonstrates its versatility by utilizing a wide range of substances. Different γ-chiral amides can be synthesized using a variety of alkenes and amines. Some examples of substrates are:
Alkenes: You can use both terminal and internal alkenes, which allows you to incorporate different functional groups.
Amines, including primary, secondary, and certain tertiary amines, can undergo the reaction, enabling the production of a diverse array of amides.
The method’s versatility in utilizing various substrates demonstrates its effectiveness in producing intricate molecules with unique structural characteristics.
Obstacles and resolutions:
The selective synthesis of γ-chiral amides using copper-catalyzed reductive relay hydroaminocarbonylation has many benefits, but it also has a lot of problems.
Substrate Compatibility: The reaction conditions may not be suitable for all alkenes and amines; thus, it is important to choose and optimize the substrates carefully.
Catalyst Stability: The copper catalyst’s capacity to remain stable during the reaction conditions may provide a constraint. Modifying ligands and employing stabilizing additives can effectively improve the performance of catalysts.
Reaction Efficiency: When trying to achieve both high yields and enantioselectivity simultaneously, reaction efficiency can be difficult. To resolve this problem, one can adjust the reaction conditions and utilize sophisticated catalytic systems.
Scientists are continuously working on finding solutions to these issues, improving the strength and usefulness of the method.
Pharmaceutical synthesis applications:
The utilization of this synthesis technique in pharmaceutical synthesis has enormous potential. Case studies demonstrate the efficacy of producing prospective medication candidates. For example:
Anti-Cancer Agents: The text’s topic is the synthesis of γ-chiral amides with cytotoxic capabilities, which are agents that can be used to treat cancer.
Antiviral Compounds: The creation of amides with antiviral characteristics prevents the spread of viruses.
Anti-Inflammatory Drugs: Researchers are developing amides that control inflammatory pathways to create anti-inflammatory medications.
These examples illustrate how the technique can simplify drug development processes, providing a pathway to create new therapeutic medicines with both high enantioselectivity and yield. Impact of leaving groups.
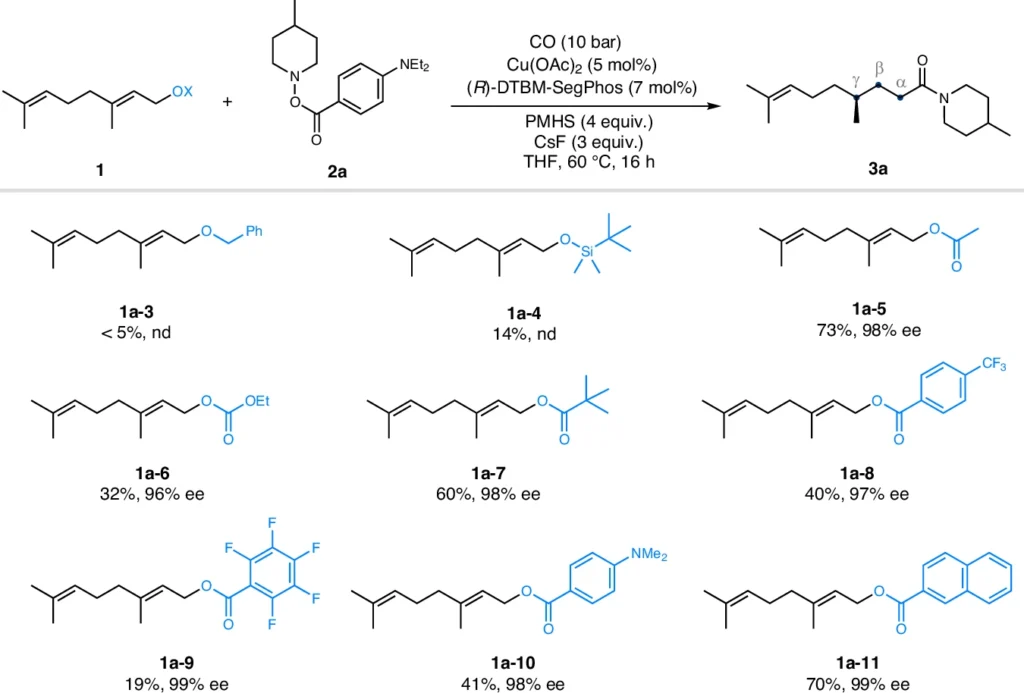
Benefits of this Approach:
Compared to conventional synthesis methods, the copper-catalyzed reductive relay hydroaminocarbonylation method has numerous advantages:
Enhanced Enantioselectivity: The use of a chiral copper catalyst guarantees elevated enantioselectivity, a critical factor in pharmaceutical applications.
Enhanced Yields: Incorporating relay and reductive components into the process improves the reaction’s efficiency, resulting in higher yields.
Economic Advantages: The use of copper as a catalyst proves to be cost-efficient, reducing the overall synthesis process’s expenditure.
Environmental Advantages: This approach frequently utilizes gentler conditions and produces less trash in comparison to conventional procedures, rendering it more ecologically sustainable.
The method’s features make it an appealing choice for large-scale synthesis in pharmaceutical manufacturing.
Prospects for the Future:
The prospects for enantioselective synthesis by copper-catalyzed reductive relay hydroaminocarbonylation seem promising. Possible research areas include:
Optimization of Reaction Conditions: Continued efforts to optimize temperature, pressure, and solvent conditions to improve the reaction’s yield and selectivity.
Expansion of Substrate Scope: Expanding the range of substrates by exploring new alkenes and amines can broaden the variety of -chiral amides available for production.
Progress on New Catalysts: Making copper catalysts better and more specific, and maybe adding new ligands and additives.
Incorporation into Continuous Flow Processes: Modifying the technique for implementation in continuous flow systems, has the potential to greatly enhance efficiency and scalability in pharmaceutical manufacture, therefore leading to a revolutionary impact.
These upcoming areas hold the potential to broaden the range of applications and influence of this synthesis technology, thereby creating new opportunities in the fields of drug discovery and chemical synthesis.
In conclusion:
Overall, copper-catalyzed reductive relay hydroaminocarbonylation is a big step forward in organic chemistry because it makes it possible to make γ-chiral amides selectively. This approach not only improves the effectiveness and specificity of amide synthesis but also shows significant potential for medicinal applications. Scientists can achieve elevated levels of enantioselectivity and yield by utilizing the distinctive characteristics of copper catalysts and the advantages of relay and reductive processes. Consequently, this technique becomes a valuable asset for synthetic chemists. We expect this method to become a fundamental technique in the production of chiral amides as research advances and discoveries emerge, thereby stimulating innovation in pharmaceutical chemistry.
Frequently Asked Questions:
1). What is enantioselective synthesis?
The process of enantioselective synthesis is very specific and only makes one enantiomer of a chemical. This is necessary for making medicines that work well and are safe.
2). What is the mechanism by which copper catalysis enhances the synthesis process?
The use of copper as a catalyst improves the synthesis procedure’s effectiveness and specificity, resulting in increased cost-effectiveness and environmental sustainability.
3). What is the purpose of using γ-chiral amides?
The pharmaceutical industry uses chiral amides as active compounds due to their unique biological properties.
4). What are the primary benefits of employing reductive relay hydroaminocarbonylation?
Compared to classic synthesis methods, this approach provides superior enantioselectivity, improves yields, and frequently exhibits greater environmental friendliness.
5). What are the future possibilities for applying this technology in the field of pharmaceutical chemistry?
Further research could improve conditions, expand the range of materials available, and integrate the approach into continuous flow processes, potentially transforming the pharmaceutical manufacturing industry.
The enantioselective synthesis of γ-chiral amides through copper-catalyzed reductive relay hydroaminocarbonylation is now more efficient, selective, and useful for making important pharmaceutical intermediates. This is made possible by the latest progress in reaction design and catalysis.
For more chemistry blogs, visit chemistry Master