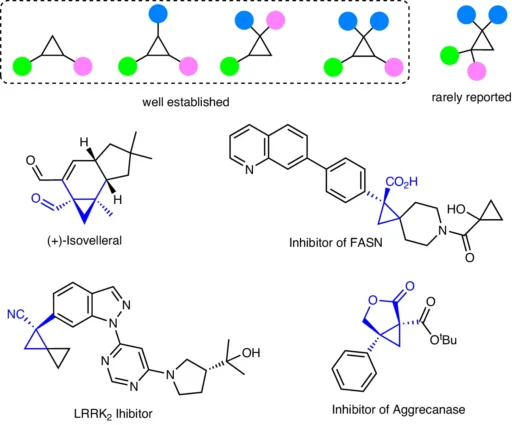
Table of Contents
The opening statement of Trisubstituted Cyclopropenes:
Hydroformylation, which is also known as the oxo process, is a basic method in organic chemistry that turns alkenes into aldehydes by adding a formyl group (-CHO) and a hydrogen atom. The reaction, commonly facilitated by transition metals like rhodium or cobalt, is essential in the production of various compounds, including fine chemicals, medicines, and agrochemicals. Trisubstituted cyclopropenes are an interesting substrate for hydroformylation because of their unique structural features and high synthetic potential. This article delves into the intricacies of chemo-, regio-, and enantioselective hydroformylation of trisubstituted cyclopropenes, with a focus on the synthesis of chiral quaternary cyclopropanes.
Understanding Hydroformylation:
Transition metals catalyze the reaction known as hydroformylation, which converts an alkene into an aldehyde by adding carbon monoxide and hydrogen. Initially identified by Otto Roelen in 1938, the process has subsequently emerged as a fundamental technique in industrial chemistry for synthesizing aldehydes from olefins. In this reaction, a rhodium or cobalt catalyst is usually used in a syngas environment comprising hydrogen and carbon monoxide. This results in the aldehyde product being produced. The substituted cyclopropane motifs and representative bioactive molecules.
Historical Context:
The hydroformylation reaction has undergone substantial advancements since its initial discovery. Originally, people primarily used cobalt catalysts due to their widespread availability and high efficiency. Nevertheless, rhodium-based catalysts have become more prominent because of their increased activity and selectivity. They can operate under less severe circumstances and produce purer products.
Catalysts Employed:
The catalysts used in hydroformylation are usually transition metal complexes, such as rhodium and cobalt. When compared to cobalt catalysts, rhodium catalysts, which are often paired with phosphine ligands, work better in terms of both activity and selectivity. Choosing the right ligands, like triphenylphosphine (PPh) or bidentate phosphines, has a big effect on how the reaction turns out. It changes both how fast and how specifically the hydroformylation process works.
Overall Mechanism:
The hydroformylation mechanism comprises multiple pivotal steps:
Coordination: The alkene binds to the metal core of the catalyst.
Migratory insertion is the process by which carbon monoxide inserts itself into the bond between a metal and an alkyl group, forming an acyl complex.
Hydrogenation: Hydrogen adds to the acyl complex, forming an aldehyde and regenerating the catalyst.
The metal catalyst assists in the iterative conversion of numerous alkene molecules into aldehydes. Control experiments and kinetic resolution reaction.
The significance of chemo-, rego-, and enantioselectivity:
Selectivity is a critical aspect of hydroformylation, determining the efficiency and specificity of the reaction. To improve the hydroformylation process, it is important to understand the different types of selectivity, which are chemo-, regio-, and enantioselectivity.
Definition of Selectivity in Chemistry:
Chemo-selectivity is the ability of one functional group to react more strongly with other possible reactive sites in the same molecule.
Regioselectivity is the tendency to favor the formation of a specific constitutional isomer when there are several other isomeric products.
Enantio-Selectivity: Chiral molecules favor one enantiomer’s production over the other. This property is essential for the production of physiologically active chemicals.
Hydroformylation’s significance:
Hydroformylation with high selectivity effectively transforms alkenes into the required aldehyde products while minimizing unwanted side reactions and byproducts. This is particularly important in complex compound synthesis because regulating the generation of specific isomers and enantiomers is critical for the compound’s biological activity and function.
Obstacles and Resolutions:
Achieving elevated levels of chemo-, regio-, and enantioselectivity in hydroformylation presents numerous challenges, including:
Substrate Complexity: The substrate’s numerous reactive sites and stereocenters can make the reaction more complex.
Catalyst Design: Developing catalysts that can selectively interact with the substrate and steer the reaction toward the desired result involves careful design and optimization.
Reaction Conditions: Optimizing temperature, pressure, and solvent conditions to maximize selectivity without compromising reaction efficiency.
These issues have been solved by improvements in catalyst design, especially the discovery of chiral ligands and more complex catalytic systems. These improvements have made hydroformylation processes very selective.
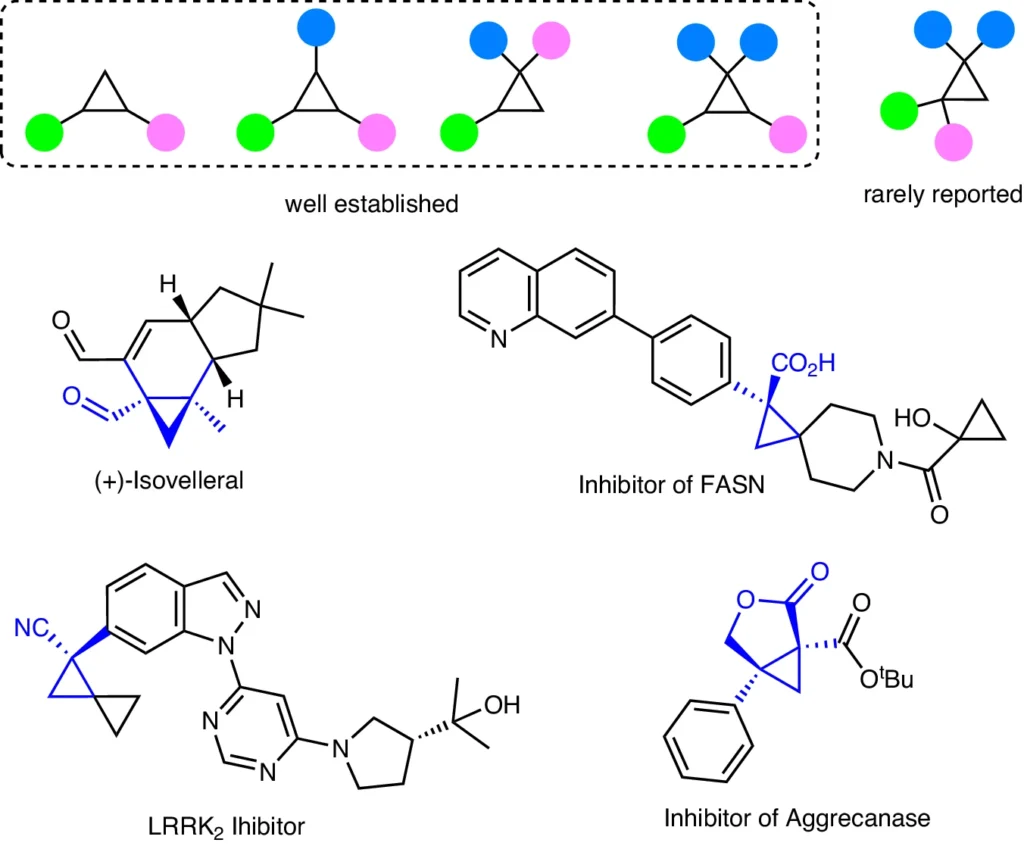
Trisubstituted Cyclopropenes:
Trisubstituted cyclopropenes are substances with a cyclopropene ring and three substituents connected to it. These compounds are highly intriguing in the field of organic synthesis because of their strained ring system and the ability to generate intricate, chiral molecules.
Chemical structures and properties:
Three carbon atoms make up the small cyclopropene ring, which has a double bond between the two of them. This ring is characterized by its high level of strain and reactivity. Three substituents on the ring can significantly influence the reactivity and selectivity of hydroformylation processes. You can modify the substituents to incorporate alkyl, aryl, or functional groups, each of which influences the reaction’s outcome.
Methods of synthesis:
The standard procedure for synthesizing trisubstituted cyclopropenes involves the following:
A cyclopropene ring can be made through cyclopropanation reactions like the Simmons-Smith reaction or metal-catalyzed cyclopropanation 1.
Introduction of Substituents: Adding or replacing different substituents to the cyclopropene ring to change its function.
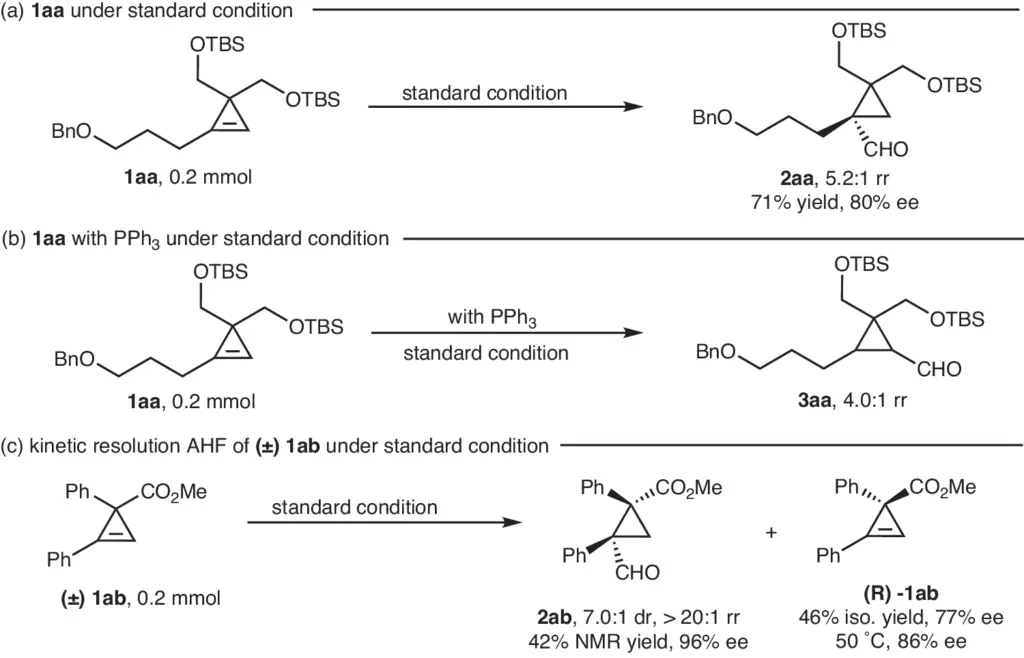
Prior studies and discoveries: Researchers who have looked into the hydroformylation of cyclopropenes with three substituents have pointed out how hard it is to get the best selectivity and productivity. Research has shown that the selection of catalyst and reaction conditions significantly influences the reaction’s outcome. For example, the use of rhodium catalysts paired with chiral ligands has shown promise for achieving elevated enantioselectivity. Scope of kinetic resolution reactions.
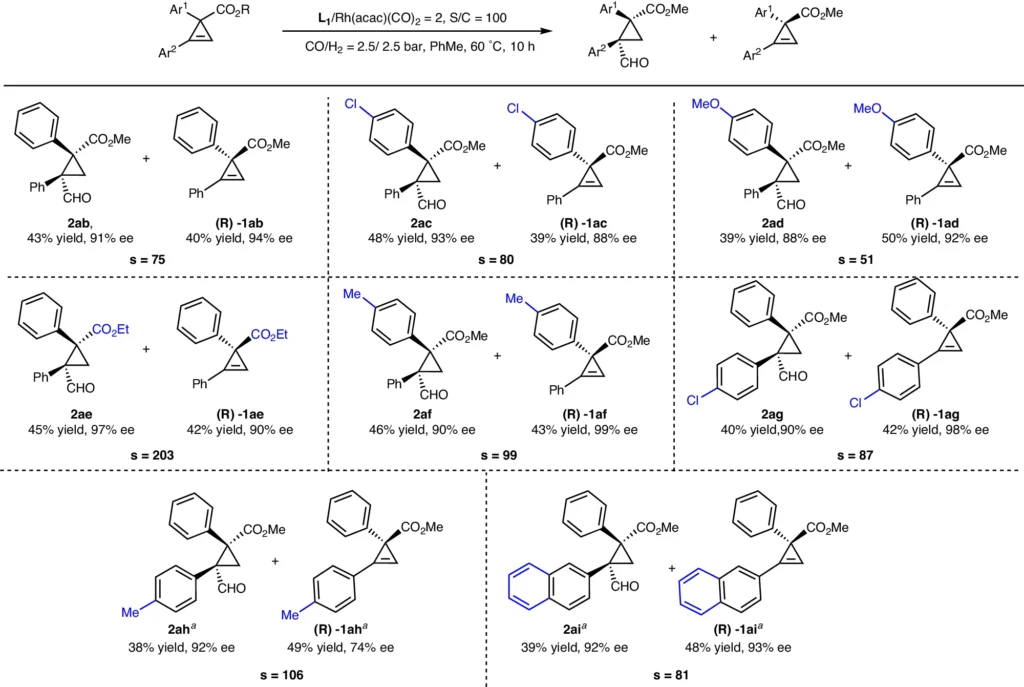
Difficulties encountered during the hydroformylation process:
The primary challenges faced during the hydroformylation of trisubstituted cyclopropenes are as follows:
steric hindrance: Large substituents can obstruct the catalyst from reaching the reactive sites, a phenomenon known as steric hindrance.
Electronic Effects: The substituents’ electronic characteristics can impact the reaction’s reactivity and selectivity.
Ring strain: The cyclopropene ring exhibits ring strain, which can lead to competing reactions and the formation of side products.
Hydroformylation Catalysts:
The catalyst selection is a critical determinant in the hydroformylation of trisubstituted cyclopropenes, influencing the reaction’s effectiveness, specificity, and production.
Categories of catalysts:
Hydroformylation catalysts commonly rely on transition metals like nickel and cobalt. People often favor rhodium catalysts in conjunction with phosphine ligands due to their superior activity and selectivity. The ligands can exist as monodentate or bidentate, with bidentate ligands offering superior control over the reaction’s selectivity.
Criteria for selection:
When choosing a catalyst for hydroformylation, it is important to take into account factors such as
Activity: The catalyst’s ability to effectively accelerate the reaction.
Selectivity is defined as the catalyst’s ability to promote the production of the intended product while minimizing the development of other by-products.
stability: The ability of a catalyst to stay active and not deactivate when exposed to reaction conditions is known as stability.
Cost: The economic viability of implementing the catalyst on a large-scale industrial level.
Efficiency in a variety of situations:
The efficacy of hydroformylation catalysts can vary considerably depending on the reaction parameters, such as temperature, pressure, and solvent. For instance, rhodium catalysts typically exhibit more efficacy in gentle conditions, whereas cobalt catalysts may necessitate elevated temperatures and pressures to attain comparable outcomes.
Advancements in Catalyst Design:
Current advancements in catalyst design have prioritized the development of chiral ligands that can augment antiselectivity. These ligands establish an imbalanced environment surrounding the catalytic site, promoting the production of one enantiomer over the other. Furthermore, progress in computational chemistry has facilitated the deliberate creation of catalysts with customized characteristics to suit particular processes. Synthetic utility.
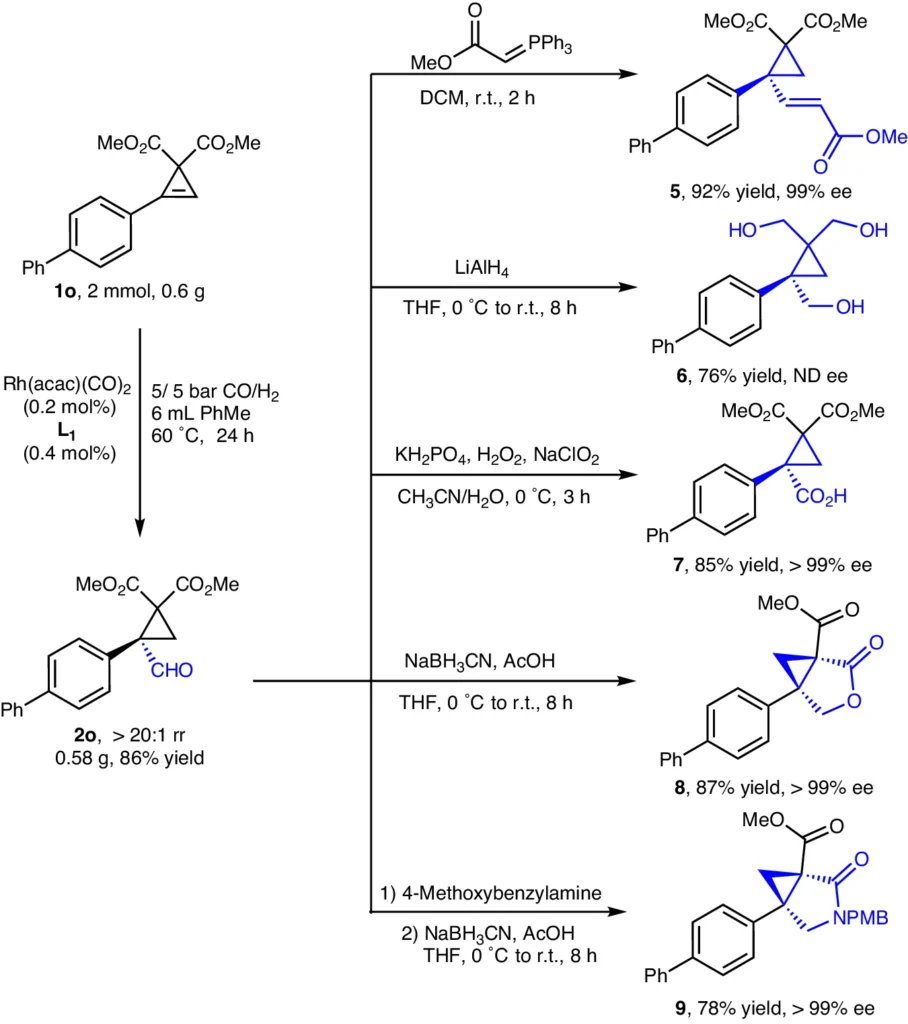
Hydroformylation mechanism of trisubstituted cyclopropenes:
The steric and electronic characteristics of the substituents regulate the hydroformylation process of trisubstituted cyclopropenes, which involves multiple crucial phases.
Sequential Analysis:
The trisubstituted cyclopropene alkene forms a coordination bond with the catalyst’s metal core.
Migratory insertion is the process by which carbon monoxide inserts itself into the bond between a metal and an alkyl group, forming an acyl complex.
The process of hydrogenation adds hydrogen to the acyl complex. This makes an aldehyde and brings the catalyst back to life.
The function of catalysts:
The catalyst expedites every stage of the reaction by supplying the required activation energy and stabilizing the intermediates. Rhodium catalysts are highly efficient at stabilizing the acyl intermediate, facilitating efficient hydrogenation and aldehyde product production.
Impact of Substituents:
What the substituents on the cyclopropene ring are made of and how they interact with space and electricity can have a big effect on the hydroformylation reaction. Large substituents can impede the alkene’s coordination with the metal center, but electron-withdrawing groups can increase the alkene’s reactivity.
Computational Studies:
Computational studies have taught us useful things about how hydroformylation works, which lets us predict and improve reaction outcomes. These studies can simulate the effects of different substituents on electrons and sterics, which can help scientists make better catalysts and reaction conditions.
Hydroformylation’s Chemo-Selectivity:
In hydroformylation, chemo-selectivity ensures that the formylation process takes place at the intended site without affecting other functional groups. Attaining a high level of chemo-selectivity is crucial to generate products that are pure and specific.
Definition and Significance:
Chemo-selectivity refers to a chemical’s ability to react selectively with one functional group while ignoring other potential reactive sites within the same molecule. In hydroformylation, it is necessary to ensure that the formylation specifically takes place at the alkene site while not causing any changes to other functional groups present on the substrate.
Attaining Chemo-Selectivity:
To achieve a high level of chemo-selectivity in hydroformylation, it is necessary to carefully choose catalysts and optimize reaction conditions. When paired with the right ligands, rhodium catalysts can improve chemo-selectivity by making a unique reaction environment that supports the preferred reaction pathway. Furthermore, the choice of solvent and temperature can influence chemo-selectivity by modulating the reactivity of various functional groups.
Illustrative instances and empirical analyses:
Case studies have shown the significance of chemo-selectivity in hydroformylation. For instance, using rhodium catalysts with bidentate ligands when hydroformylation substrates with numerous reactive sites promotes the formylation of the alkene group while reducing unwanted reactions at other functional groups.
Industrial Applications:
The industry widely uses chemo-selective hydroformylation, particularly for producing high-quality chemicals and pharmaceuticals. By selectively formulating certain functional groups, this approach can achieve high-purity products with few byproducts, eliminating the need for expensive purification stages.
Regio-Selectivity in Hydroformylation:
Regioselectivity refers to the preference for a chemical reaction to occur at a specific position on a molecule. Hydroformylation is defined as the selective addition of a carbonyl group and a hydrogen atom.
Regioselectivity is very important in hydroformylation, especially for trisubstituted cyclopropenes because they can have more than one possible regioisomer. A reaction’s regioselectivity determines the precise location on the substrate to introduce the formyl group.
Definition and Significance:
Regio-selectivity is the tendency to favor the formation of a specific constitutional isomer when there are several other isomeric products. In hydroformylation, this refers to the promotion of the attachment of the formyl group to a particular carbon atom in the alkene.
Attaining Regioselectivity:
To achieve a high level of regioselectivity in hydroformylation, the catalyst and reaction conditions must be optimized to promote the creation of the desired regioisomer. The choice of catalyst, specifically the ligands employed, can have a significant impact on regioselectivity by providing stability to specific intermediates and transition states. You can also direct the formylation process to certain spots by changing the steric and electronic properties of the substituents on the cyclopropene ring.
Illustrative instances and empirical analyses:
Case studies have demonstrated the significance of regioselectivity in hydroformylation. For instance, we have demonstrated that employing rhodium catalysts with sterically demanding ligands during hydroformylation of trisubstituted cyclopropenes with large substituents promotes formylation at the position with the least steric hindrance. This leads to a high degree of selectivity in terms of the reaction’s regiochemistry.
Industrial Applications:
Regio-selective hydroformylation is critical for industrial applications, particularly in the synthesis of intricate structures where the placement of functional groups can greatly influence the result’s characteristics and reactivity. Chemists can generate specific isomers that possess desirable features by manipulating regioselectivity, thereby improving the effectiveness and usefulness of the hydroformylation process.
Enantioselectivity in hydroformylation:
In the pharmaceutical industry and other fields where the three-dimensional configuration of atoms influences biological activity, enantioselectivity plays a critical role in the production of chiral compounds with high purity.
Definition and Significance:
In chiral compounds, enantioselectivity occurs when one enantiomer forms in greater amounts than the other. Hydroformylation promotes the production of a specific enantiomer of the aldehyde product, which is critical for applications where chirality plays a significant role in biological activity and function.
Attaining Enantioselectivity:
To get a high level of selectivity for one enantiomer over the other in hydroformylation, chiral ligands must be used in processes that are catalyzed by rhodium. These ligands establish an imbalanced environment surrounding the catalytic site, promoting the production of one enantiomer more than the other. The choice of a chiral ligand and the conditions under which a reaction takes place can have a significant impact on the ability to selectively produce one enantiomer over the other. Computational studies offer valuable information for designing chiral catalysts with optimal performance.
Illustrative instances and empirical analyses:
Case studies have shown that chiral ligands can help get a high level of enantioselectivity in the hydroformylation process. For example, rhodium catalysts combined with chiral phosphine ligands have demonstrated the ability to generate chiral quaternary cyclopropanes with exceptional anti-selectivity, resulting in high optical purity products.
Industrial Applications:
Several industries widely use enantio-selective hydroformylation, with notable applications in the pharmaceutical sector. This process is particularly important in drug development, as the chirality of pharmaceuticals can greatly influence their effectiveness and safety. This approach can improve the quality and performance of pharmaceutical products by creating chiral compounds with a high degree of anti-selectivity.
Methodology:
To make sure that the right aldehyde products are made, the experimental protocols for the hydroformylation of trisubstituted cyclopropenes include slow and careful steps.
Experimental Procedures:
Catalysts use rhodium complexes with bidentate phosphine ligands.
The solvents used are toluene, dichloromethane, and ethanol.
Chemicals: trisubstituted cyclopropenes, syngas (a mixture of carbon monoxide and hydrogen gas).
Method:
Preparation: To initiate the process, dissolve the trisubstituted cyclopropene in the selected solvent.
Catalyst Addition: Introduce the rhodium catalyst and ligand into the solution.
Reaction Setup: Introduce a mixture of carbon monoxide and hydrogen gas (syngas) into the reaction vessel while maintaining a carefully regulated environment.
Reaction Conditions: Keep the reaction at the specified temperature and pressure while monitoring its progress using gas chromatography.
Product Isolation: Separate and purify the product using conventional methods like column chromatography or recrystallization.
Methods of analysis:
We employ methods like NMR spectroscopy, mass spectrometry, and chiral HPLC to analyze the products and determine their purity and enantiomeric excess. These procedures offer comprehensive data regarding the chemical structure and composition of the hydroformylation products.
There are safety-related factors to consider.
Ensuring safety is of utmost importance in hydroformylation operations, especially when dealing with syngas and transition metal catalysts. To reduce risks, follow standard laboratory safety measures such as the use of fume hoods, personal protective equipment, and proper waste disposal.
Results and Discussion:
We examine the hydroformylation test results to determine the efficiency, selectivity, and yield of the process. The results shed light on the response mechanism and the factors that influence selectivity.
Analysis of Experimental Data:
We assess experimental data such as reaction yields, selectivity ratios, and enantiomeric excess to evaluate the hydroformylation process’s performance. Conducting comparative research using various catalysts and reaction circumstances aids in determining the most favorable conditions for reaching a high level of selectivity and yield.
Analysis and explanation of findings:
We analyze the results within the framework of the reaction mechanism, taking into account the impact of substituents and catalysts. We can use the findings to guide the development of more efficient hydroformylation methods by understanding the parameters that influence chemo-, regio-, and enantioselectivity.
Comparative analysis with prior research:
We compare the results with previous studies to identify trends and advancements in the hydroformylation of trisubstituted cyclopropenes. This comparison serves to authenticate the experimental findings and emphasizes areas that require additional research and enhancement.
The implications for the field of synthetic chemistry are significant.
The study’s findings have important implications for synthetic chemistry, specifically the advancement of effective and discriminating techniques for synthesizing intricate structures. Other hydroformylation reactions and similar synthetic procedures can benefit from the knowledge this research has acquired.
Uses of Chiral Quaternary Cyclopropanes:
Chiral quaternary cyclopropanes are highly useful components in organic synthesis, playing a crucial role in the development of medicines, agrochemicals, and materials research.
Significance in the field of pharmaceuticals
Chiral quaternary cyclopropanes play a crucial role in pharmaceutical production, serving as frameworks for medications that have improved biological activity and selectivity. Their distinctive structural characteristics enable the creation of innovative therapeutic agents with enhanced effectiveness and safety profiles.
Application in the field of agrochemicals
In the field of agrochemicals, we employ chiral quaternary cyclopropanes to produce pest control agents that exhibit great specificity and minimize their environmental impact. Their distinct characteristics facilitate the creation of chemicals that selectively target pests while reducing damage to non-target species.
The field of Material Science holds significant potential.
Chiral quaternary cyclopropanes hold significant potential in the field of material science, particularly in the development of innovative materials that display unique characteristics. We can utilize their inherent structural properties to manufacture materials with precise mechanical, optical, or electrical attributes.
We analyze and study real-life situations to understand and learn from them.
Case examples demonstrate the wide-ranging uses of chiral quaternary cyclopropanes in many industries. For example, the production of a chiral pharmaceutical compound using a quaternary cyclopropane framework has improved its pharmacokinetic characteristics and therapeutic effectiveness. Rh-catalyzed asymmetric hydroformylation of different alkenes.
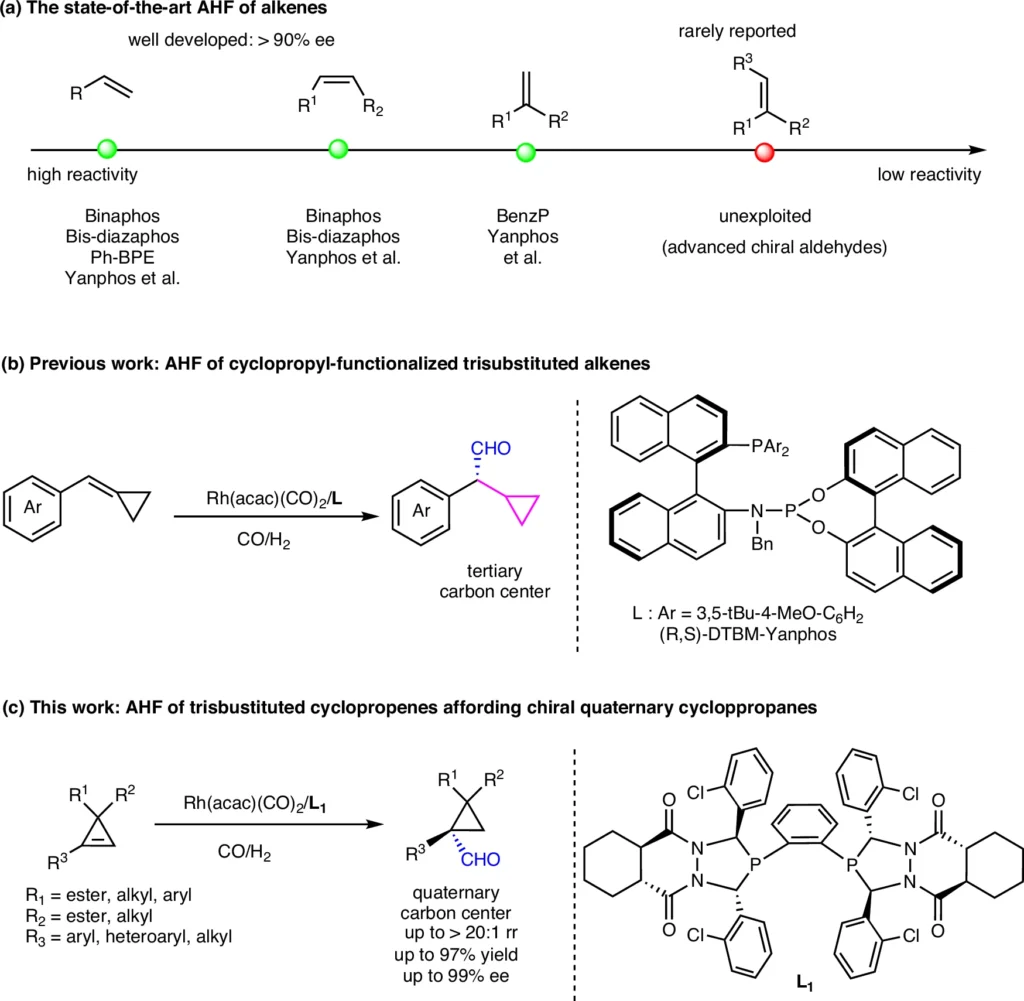
Obstacles and Prospects for the Future:
Despite significant advancements in the hydroformylation of cyclopropenes with three substituents, several obstacles remain. Further research is necessary to tackle these limits and determine future paths for investigation.
Existing Constraints:
One problem with hydroformylation of trisubstituted cyclopropenes right now is that very selective catalysts and reaction conditions are needed to get the desired selectivity. In addition, the process of creating trisubstituted cyclopropenes might be difficult, which restricts the number of available substances for hydroformylation.
Possible resolutions:
Possible resolutions to these difficulties involve the creation of catalysts that are more adaptable and resilient, capable of functioning in a broader spectrum of circumstances. The use of ligand design and computational chemistry can assist in the logical development of catalysts with enhanced selectivity and efficiency. In addition, investigating other methods of synthesizing trisubstituted cyclopropenes can broaden the range of substances that can undergo hydroformylation.
Prospects for Future Research:
Possible areas for future investigation include:
Catalyst Development: Creating novel catalysts with improved selectivity and activity.
Mechanistic Studies: Conducting in-depth investigations into the precise mechanics of hydroformylation to obtain a more profound understanding of the elements that impact selectivity.
Substrate Scope: Increasing the number of substrates that can be hydroformylation efficiently so that it can work with more complex and different molecules.
Sustainable methods: Advancing the development of hydroformylation methods that are both ecologically benign and sustainable by incorporating renewable feedstocks and utilizing green solvents.
Current developments:
For example, new developments in hydroformylation research include using catalysts that are based on biological systems and adding hydroformylation to other synthetic processes to make them more efficient and effective. Furthermore, the progress in automation and high-throughput screening techniques helps expedite the process of identifying and refining new catalysts and reaction conditions.
In conclusion:
The hydroformylation of cyclopropenes with three substituents is an interesting problem in the field of synthetic chemistry. It also opens up a lot of possibilities for making chiral cyclopropanes with four substituents. By meticulously choosing catalysts and controlling reaction conditions, it is feasible to attain elevated degrees of chemo-, regio-, and enantioselectivity. This work highlights the significance of ongoing research in this domain to discover new applications and improve the efficiency of these reactions. This research’s knowledge can guide improving hydroformylation processes, resulting in increased efficiency and selectivity. This, in turn, can lead to breakthroughs in medicines, agrochemicals, and material science.
Frequently Asked Questions:
1). Hydroformylation is a chemical reaction in which carbon monoxide and hydrogen are added to an unsaturated compound to form an aldehyde.
Hydroformylation is a chemical process in which a formyl group (-CHO) and a hydrogen atom are added to an alkene, usually with the aid of a transition metal catalyst.
2). Selectivity is important in hydroformylation because it allows for precise control over the formation of specific products.
Selectivity guarantees the formation of the intended product while minimizing the production of unwanted byproducts, thereby improving the reaction’s efficiency and usefulness.
3). Trisubstituted cyclopropenes are molecules with three substituents attached to the carbon atoms of the cyclopropene ring.
Trisubstituted cyclopropenes are cyclopropene rings with three substituents attached. These rings are characterized by their stretched structure and exhibit a wide range of chemical properties.
4). What are the uses for chiral quaternary cyclopropanes?
Medicines, agrochemicals, and material science all use these compounds as essential building blocks for a variety of applications.
5). What are the prospects for advancement in this field?
Subsequent investigations could prioritize the development of catalysts with greater adaptability, the exploration of alternative mechanisms for reactions, and the broadening of the range of cyclopropenes included in the study.
For more chemistry blogs, visit chemistry Master