Table of Contents
Recent progress in hydrogen-bonded organic frameworks (HOFs) in materials science has shown promising opportunities to enhance proton conductivity using the unique method of vitrification. This article examines the impact of vitrification on improving proton conductivity in HOFs, as well as its implications for various applications.
An Overview of Vitrification and Proton Conductivity:
What is Vitrification? Vitrification is a process that involves converting a substance into a glass-like state by rapid cooling. Vitrification is the rapid cooling process that transforms a substance into a solid state, like glass, without any crystalline structure. The preservation of biological specimens and the creation of amorphous materials with distinctive characteristics commonly employ this approach.
Importance of Proton Conductivity in Materials:
This article discusses the significance of proton conductivity in materials.
Proton conductivity is essential for a wide range of applications, such as fuel cells, electrolyzers, and sensors. It enables the efficient transportation of protons through materials, thereby allowing these devices to work.
Hydrogen-Bonded Organic Frameworks (HOFs):
Definition and Structure of HOFs: HOFs, or higher-order functions, are defined as functions that can take other functions as arguments or return functions as results. These are fundamental concepts in functional programming. HOFs are distinguished by their ability to perform functions, making them first-class citizens. This implies that variables can assign functions.
Hydrogen-bonded organic frameworks (HOFs) are a specific type of porous crystalline material consisting of organic molecules interconnected through hydrogen bonds. These frameworks exhibit a well-organized structure with empty areas that can house guest molecules.
Role of Hydrogen Bonds in HOFs:
The significance of hydrogen bonds in hydrogen-bonded organic frameworks (HOFs)
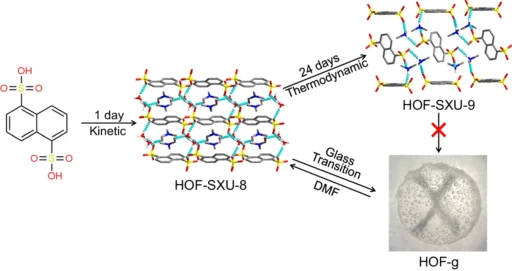
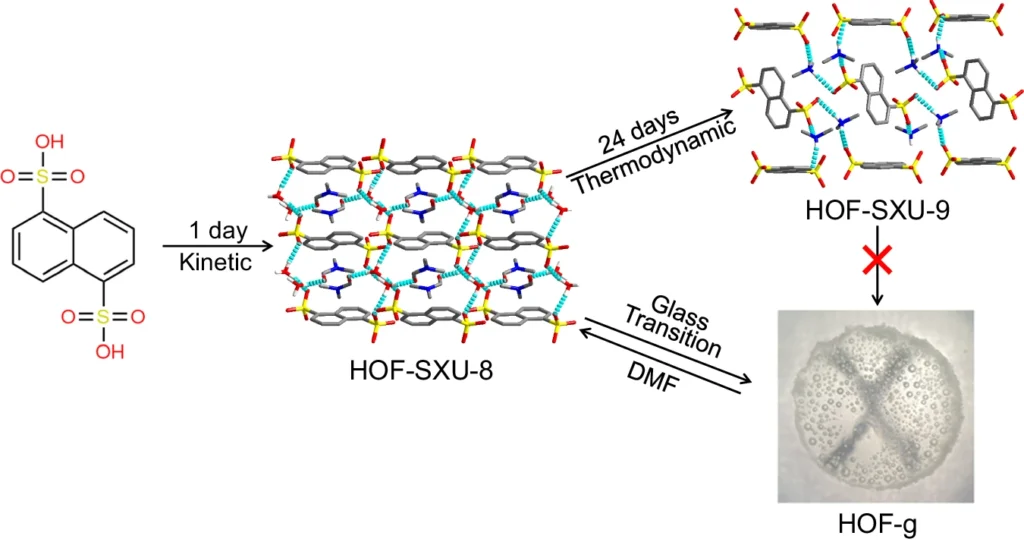
Hydrogen bonds are essential for preserving the stability and arrangement of HOFs. The hydrogen atoms form non-covalent connections with electronegative elements like oxygen, nitrogen, or fluorine, resulting in a network that provides stability to the framework. Schematic representation of the synthesis of HOF-SXU-8, HOF-SXU-9, and the vitrification transformation process to HOF-g.
Enhancing proton conductivity via vitrification:
Recent studies have shown that vitrification can greatly improve the ability of hydrogen-bonded organic frameworks to conduct protons. By subjecting HOFs to vitrification, researchers have successfully enhanced proton mobility. This breakthrough opens up new possibilities for utilizing these materials in applications such as proton exchange membranes.
Summary of Research Findings:
Researchers have found that vitrified high-ohmic fibers (HOFs) are better at conducting protons than non-vitrified HOFs. The material’s amorphous and glass-like properties, which facilitate easier movement of protons, are credited for the improvement.
The impact of vitrification on proton conductivity:
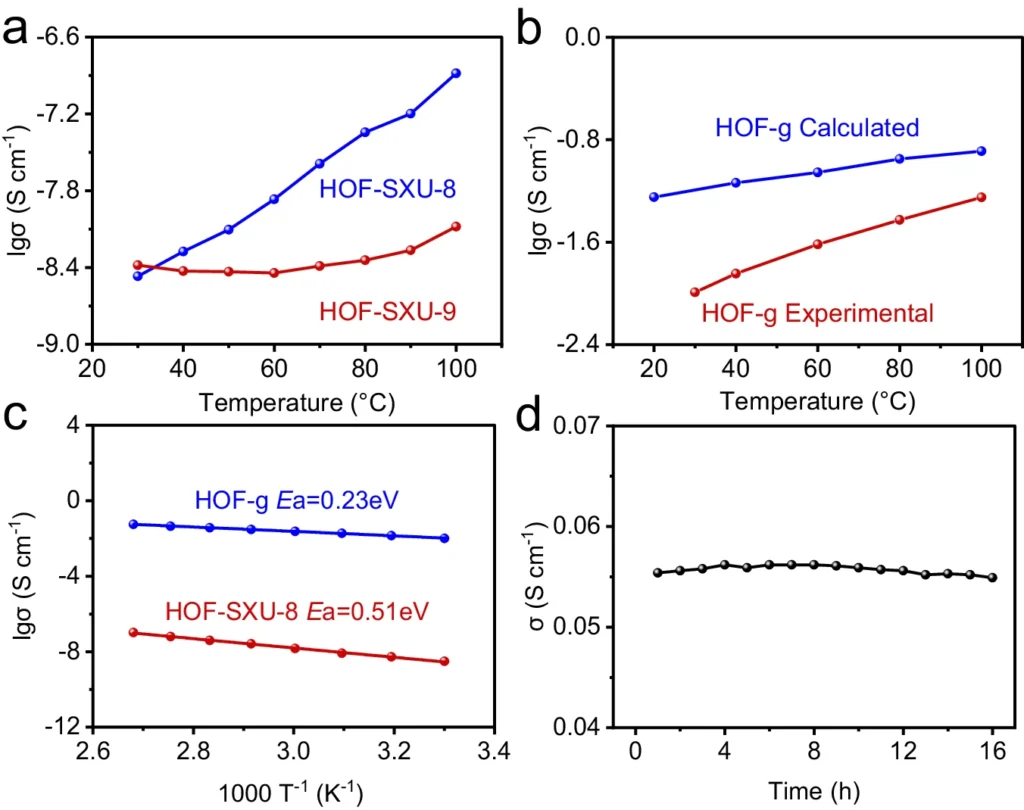
Vitrification changes the immediate surroundings in high-occupancy frameworks (HOFs), which reduces the obstacles to protons’ movement. By enabling faster and more efficient movement of protons between sites connected by hydrogen bonds, this modification enhances protons’ ability to conduct electricity.Proton conductivity property of HOF-SXU-8, HOF-SXU-9 and HOF-g.
Mechanisms Behind Proton Conductivity in Vitrified HOFs:
Explanation of Proton Transport in HOFs: Understanding Proton Conductivity in Solidified Hydrogen-Bonded Organic Frameworks
The process of moving or transferring protons inside the structure of HOFs is known as proton transport.
In hydrogen-bonded organic frameworks, protons move along paths made by hydrogen bonds inside the framework. This is called proton transport. Hydrogen bonds provide a network of routes that conduct protons.
Impact of Vitrification on These Mechanisms: Vitrification disrupts the crystalline structure of highly ordered frameworks (HOFs), resulting in their transformation into a less organized, amorphous phase. This disorder decreases the obstacles for proton transport and facilitates unimpeded proton diffusion through the material.
The development of vitrified high-ohmic films (HOFs) with improved proton conductivity has the potential for a variety of applications, including proton exchange membranes used in fuel cells and electrolyzers. The proton conduction mechanism of HOF-g.
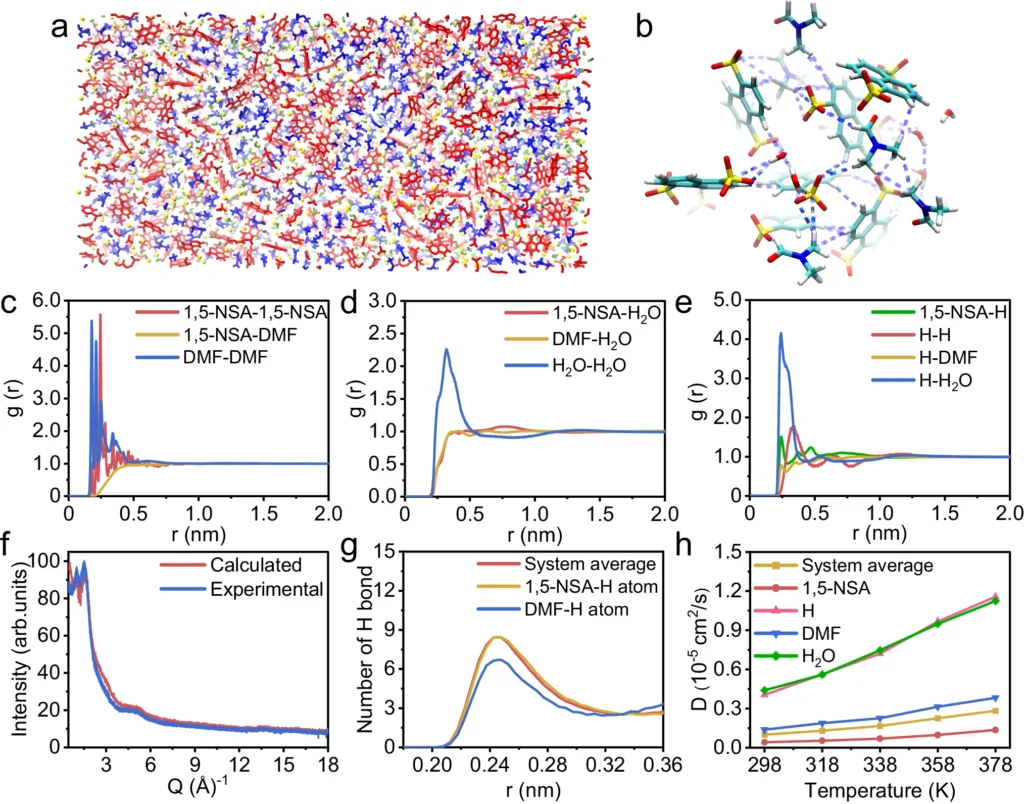
Possible Applications for Proton Exchange Membranes:
Potential Uses in Proton Exchange Membranes: Because of their exceptional proton conductivity and durability, vitrified HOFs are highly suitable for use as efficient proton exchange membranes in a variety of operating conditions. The membranes are critical in fuel cells because they allow protons to move through the electrolyte.
Benefits Compared to Traditional Materials:
Vitrified HOFs provide various advantages when compared to typical proton-conducting materials such as perfluorosulfonic acid membranes. These materials demonstrate enhanced proton conductivity at elevated temperatures and in humid environments, rendering them better suited for challenging applications.
Prospects and Areas for Future Study:
The discovery that vitrification can improve the conductivity of protons in organic frameworks that are hydrogen-bonded opens up new ways to study this topic and make progress.
Topics requiring additional research and improvement:
Future research should prioritize the refinement of vitrification methods to customize the characteristics of HOFs for specific uses. By understanding the basic steps involved in the movement of protons in vitrified hydrogen-bonded organic frameworks (HOFs), we might be able to come up with higher-performing materials.
The Significance of Advancing Vitrified High-Order Function Technology:
To get the most out of these materials in proton conductivity applications, we need to develop vitrified high-temperature oxide fuel (HOF) technology. This includes investigating innovative synthesis techniques, analyzing the relationship between structure and properties, and expanding production processes.
In conclusion:
Overall, vitrification is a revolutionary method for improving proton conductivity in hydrogen-bonded organic frameworks. This new method makes it easier to make non-crystalline materials that are better at moving protons around. This could lead to progress in technologies that convert and store energy.
Commonly Asked Questions (FAQs):
1). What is the mechanism by which vitrification improves proton conductivity in hydrogen-bonded organic frameworks (HOFs)?
Vitrification disrupts the crystalline structure of HOFs, creating a disordered environment that promotes accelerated proton transport.
2). What are some of the potential applications of vitrified high-ohmic fibers (HOFs) in fuel cell technology?
Vitrified high-temperature organic frameworks (HOFs) have the potential to serve as proton exchange membranes in fuel cells, thereby enhancing both efficiency and durability.
3). What makes hydrogen-bonded organic frameworks (HOFs) a suitable choice for proton conductivity?
Hollow organic frameworks (HOFs) have an adjustable arrangement and a unique network of hydrogen bonds that facilitate the efficient movement of protons.
4). What are the challenges that must be addressed when expanding the use of vitrification processes in industrial applications?
To expand vitrification processes, it is necessary to enhance the process for mass manufacturing while ensuring the preservation of material integrity and performance.
5). What impact does vitrification have on the stability and durability of proton exchange membranes?
Vitrification improves the thermal and chemical durability of proton exchange membranes, hence increasing their longevity.
For more chemistry blogs, visit chemistry Master