Table of Contents
Overview of Hydrogen Oxidation:
Hydrogen has emerged as a leading contender for future energy solutions due to its potential to provide cleaner and more efficient energy sources. Hydrogen’s potential as an environmentally friendly energy carrier lies in its ability to generate electricity in fuel cells, producing only water as a byproduct. However, hydrogen-based technologies depend a lot on how well the catalytic processes work, especially the hydrogen oxidation reaction (HOR) and the hydrogen evolution reaction (HER).
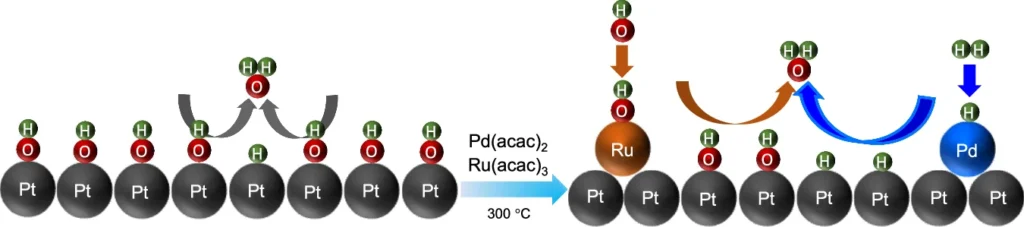
Devices like fuel cells and electrolyzers, which either use hydrogen to generate power or produce it from water, rely heavily on these reactions. Although platinum (Pt) catalysts have demonstrated outstanding efficacy in acidic conditions, they frequently encounter difficulties in alkaline settings, resulting in a considerable decrease in reaction kinetics. This article explores the novel application of a palladium-ruthenium (Pd-Ru) pair on a platinum surface. This mix might get around these problems and make the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER) work better in alkaline environments. Hydrogen oxidation reaction schematic diagram on Pt and Pd-Ru@Pt surface.
Understand the processes of hydrogen oxidation and evolution reactions (HOR/HER):
To completely comprehend the importance of the Pd-Ru/Pt catalyst, it is crucial to have a thorough understanding of the underlying chemical interactions involved. The hydrogen oxidation reaction (HOR) is a chemical reaction in which hydrogen molecules undergo electrolysis, separating protons and electrons. Fuel cells rely on this reaction to function, using the produced electrons to generate electricity.
Conversely, the Hydrogen Evolution Reaction (HER) is the process of combining protons and electrons to create hydrogen gas, which is essential in electrolyzers used for producing hydrogen. These reactions are electrochemical and require catalysts to reduce energy barriers, allowing the reactions to occur at a reasonable speed.
Difficulties with Hydrogen Oxidation Reactions in Alkaline Media:
Although HOR and HER exhibit relatively efficient performance in acidic environments using platinum-based catalysts, their behavior is distinct in alkaline environments. Elevated pH levels, a common indicator of highly basic settings, present numerous challenges. One of the most important factors is the reduced speed at which protons travel, resulting in increased overpotentials. Overpotentials refer to the extra voltage required to push processes beyond their thermodynamic potential. As a result, efficiency decreases, hindering the attainment of the specified reaction rates without excessive energy consumption.
The role of catalysis in the hydrogen oxidation reaction (HOR) and the hydrogen evolution reaction (HER) is significant:
Catalysts are substances that accelerate a chemical reaction without depleting themselves in the process. In homogeneous oxidation reactions (HOR) and heterogeneous electrochemical reactions (HER), the catalyst facilitates the attraction and separation of hydrogen molecules on its surface, as well as the subsequent movement of protons and electrons. We assess a catalyst’s efficacy by its ability to lower the energy barriers of these phases, thereby increasing the overall reaction rate.
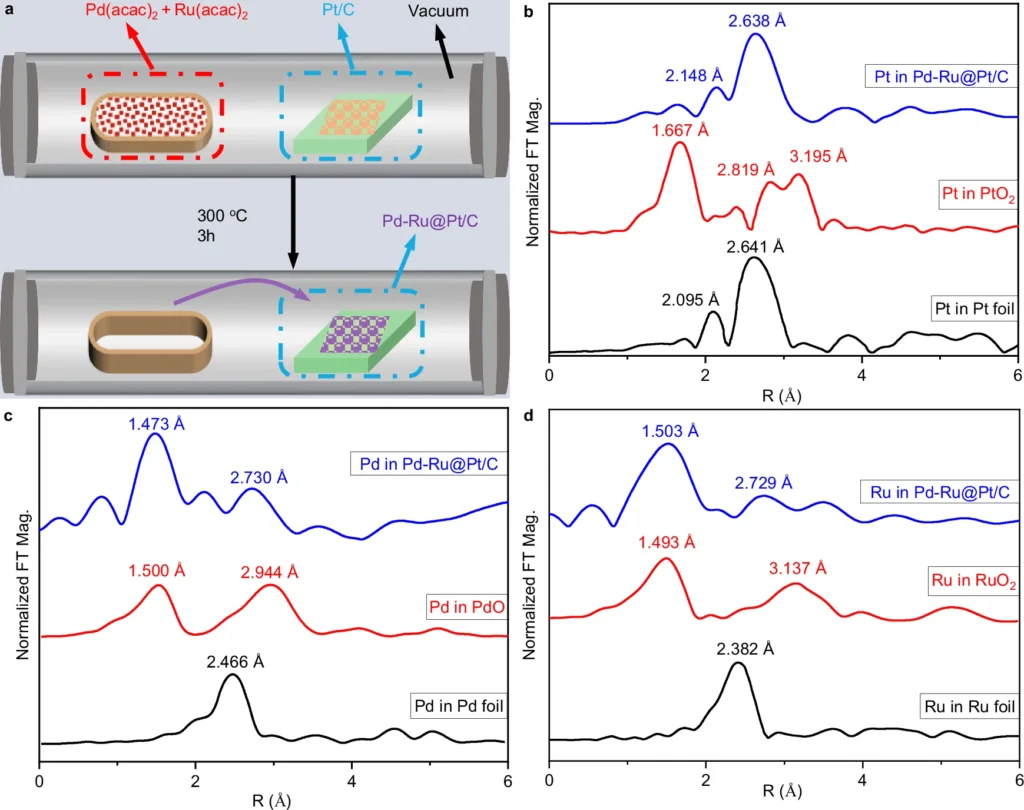
When dealing with alkaline environments, where the speed of chemical reactions is naturally slower, the catalyst’s careful design becomes even more crucial. An optimally engineered catalyst can substantially decrease the overpotential, thereby enhancing the efficiency of the reaction and diminishing the energy expenditure. Preparation and structure characterization of electrocatalysts.
What are the advantages of using a Pd-Ru pair?
Palladium (Pd) and ruthenium (Ru) are transition metals that possess desirable characteristics for catalytic applications. Palladium is renowned for its exceptional capacity to absorb and dissolve hydrogen molecules. This property makes it highly efficient in hydrogen-based processes. Ruthenium is well-known for its stability, resistance to corrosion, and ability to allow proton exchange.
The combination of Pd and Ru results in a synergistic effect, where the individual strengths of each metal enhance and support one another. These two reactions work better together to attract and activate hydrogen molecules, which is necessary to speed up the hydrogen oxidation reaction (HOR) and the hydrogen evolution reaction (HER). Furthermore, the contact between the Pd-Ru pair and the Pt atoms beneath increases the catalytic activity of the Pd-Ru pair when supported on a Pt surface.
What is the significance of platinum (Pt) in catalysis?
Platinum has traditionally served as the standard for catalysts, especially in processes involving hydrogen. The great efficacy of this substance in both the hydrogen oxidation reaction (HOR) and the hydrogen evolution reaction (HER) is due to its capacity to adsorb hydrogen atoms and assist their conversion into protons and electrons, or vice versa. Nevertheless, platinum encounters difficulties when exposed to alkaline solutions, as the reaction rates might be slow.
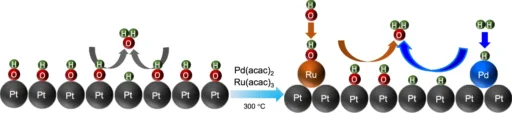
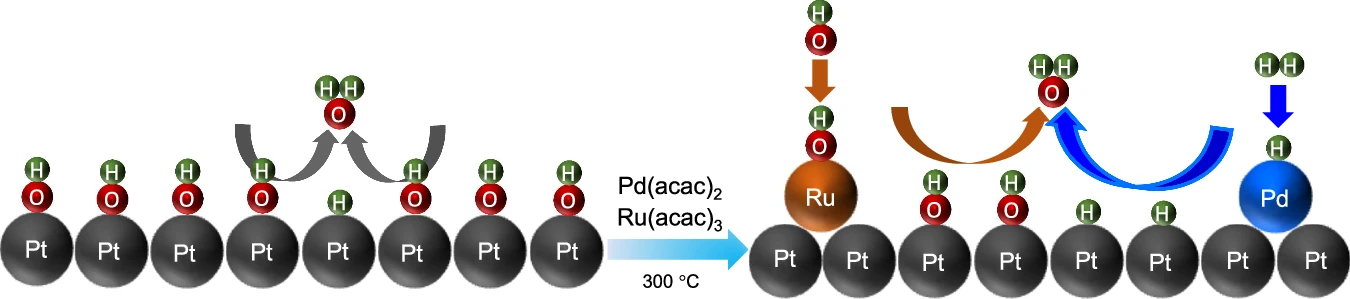
To circumvent these constraints, researchers intend to introduce a Pd-Ru pair onto the Pt surface. Incorporating Pd and Ru not only promotes hydrogen adsorption and dissociation but also increases the catalyst’s overall stability. This combination results in the formation of a catalytic surface that exhibits enhanced activity and increased resistance to deactivation, resulting in superior performance in alkaline environments. Time evolution and structural dynamics of the Pd-Ru@Pt (110) slab.
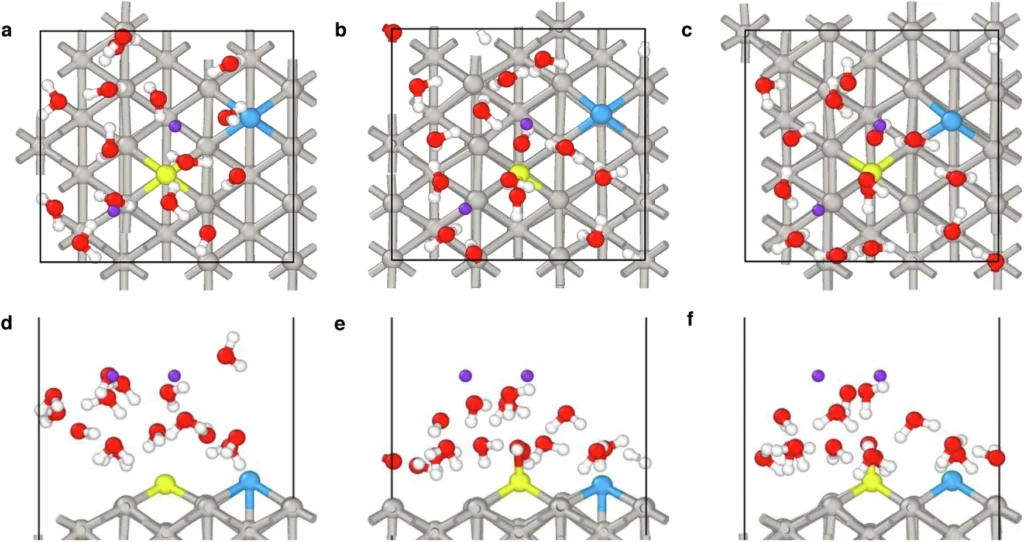
On a platinum (Pt) surface, create the Pd-Ru combination:
The integration of a Pd-Ru pair onto a Pt surface necessitates meticulous engineering to ensure that the metals are deposited in an ideal arrangement. We commonly employ methods like electrodeposition, chemical vapor deposition (CVD), or physical vapor deposition (PVD) to produce a thin and even layer of Pd-Ru on the Pt surface.
We aim to achieve a configuration that uniformly disperses the Pd and Ru atoms, thereby optimizing the accessibility of active sites. The active sites are crucial for successful catalysis, as these are the locations where hydrogen molecules will adsorb and dissociate. We meticulously engineer the Pd-Ru/Pt surface to possess specific structural and chemical features, ensuring optimal performance of the catalyst in alkaline media throughout the HOR and HER processes.
Understanding the Mechanism of Hydrogen Oxidation and Reduction Promotion by Palladium-Ruthenium on Platinum:
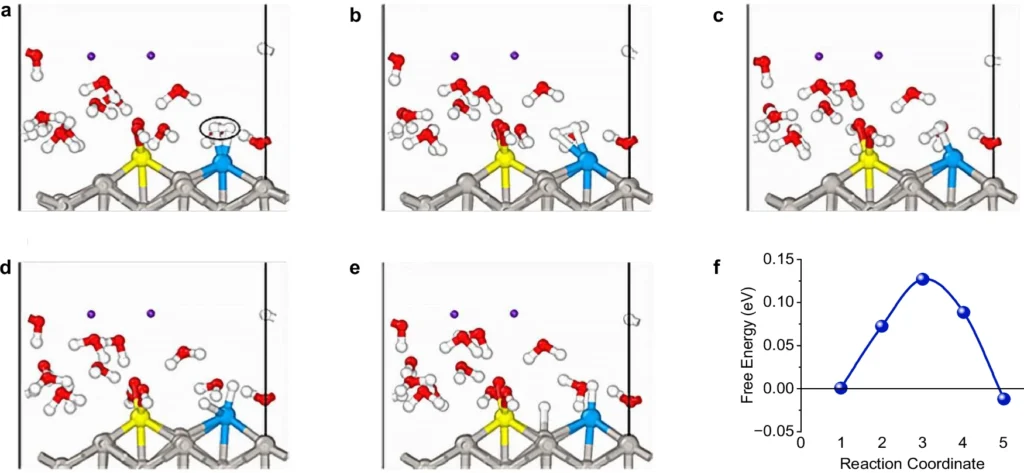
The Pd-Ru/Pt catalyst improves the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER) by employing a variety of processes simultaneously. Palladium, due to its high affinity for hydrogen, serves as the main location for hydrogen adsorption. Once adsorbed, the hydrogen molecules dissociate into atoms, with the ruthenium atoms stabilizing them. Ruthenium is very important because it helps move protons and electrons around, which is needed for the hydrogen oxidation reaction (HOR) or hydrogen evolution reaction (HER) to finish.
This procedure effectively reduces the energy barriers linked to the reaction steps, resulting in a decrease in overpotential. Put simply, the catalyst facilitates the reaction by reducing the energy required, thereby enhancing the system’s efficiency.
Electrochemical performance in alkaline media:
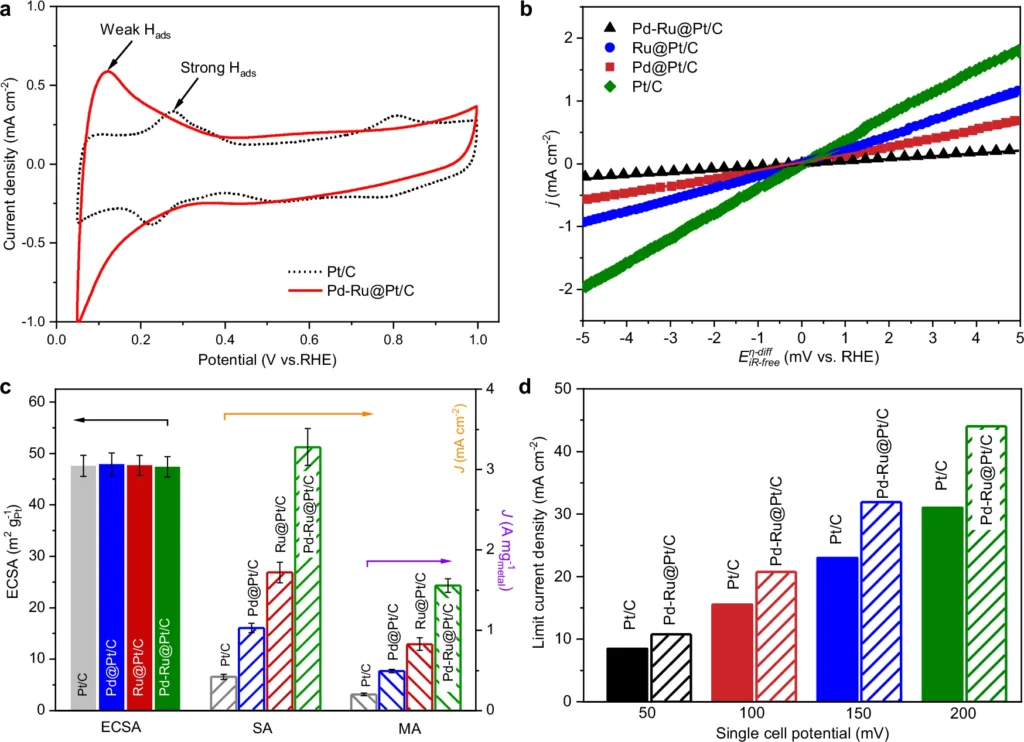
Researchers have conducted extensive research to analyze and compare the performance of the Pd-Ru/Pt catalyst with standard Pt catalysts. The Pd-Ru/Pt catalyst routinely demonstrates superior performance compared to Pt alone in alkaline environments. Various crucial indicators demonstrate this.
Reduced Overpotentials: The Pd-Ru/Pt catalyst necessitates a smaller amount of extra voltage to facilitate the reactions, resulting in enhanced efficiency.
Higher current densities indicate increased catalyst activity because they digest more hydrogen at a given voltage.
Improved Stability: The Pd-Ru/Pt catalyst exhibits enhanced resistance to deactivation, allowing it to sustain its performance for extended durations.
Because of the improvements, the Pd-Ru/Pt catalyst is a very good choice for use in alkaline fuel cells and electrolyzers, where long-term stability and efficiency are very important. Electrochemical performance and mechanism of different catalysts.
Benefits of using a Pd-Ru catalyst instead of Pt for the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER):
Integrating a Pd-Ru pair onto a Pt surface has numerous benefits for Hydrogen Oxidation reaction compared to conventional catalysts. The following items are included:
Enhanced Catalytic Efficiency: The combined influence of Pd and Ru generates a greater number of active sites for hydrogen reactions, resulting in accelerated reaction rates and reduced energy consumption.
Enhanced Stability: The Pd-Ru/Pt catalyst exhibits greater resistance to typical problems such as deactivation and corrosion, thereby guaranteeing a prolonged and more durable performance.
Versatility: The improved efficiency of the Pd-Ru/Pt catalyst in alkaline environments makes it well-suited for a broader spectrum of uses, including fuel cells and electrolyzers.
Practical uses and consequences in the real world:
The advancements in Pd-Ru/Pt catalysis have significant implications for the hydrogen economy. This catalyst has the potential to decrease the overall cost of hydrogen production and utilization by enhancing the efficiency and durability of HOR and HER. It is crucial for the advancement of fuel cells and electrolyzers, which are pivotal technologies in the shift towards a sustainable energy future.
The utilization of the Pd-Ru/Pt catalyst in fuel cells can enhance power outputs and extend operating lifetimes, hence increasing the feasibility of hydrogen-powered vehicles and electronics. Enhanced efficiency in electrolyzers can reduce the costs associated with producing green hydrogen, thereby increasing its competitiveness relative to fossil fuels.
Obstacles and Prospects for the Future:
Despite the potential of the Pd-Ru/Pt catalyst, there are still obstacles to overcome before its widespread use. One of the primary obstacles is the cost of the materials required. Palladium, ruthenium, and platinum are all very costly, which may hinder the economic feasibility of employing this catalyst on a significant scale.
Scalability presents an additional challenge. Manufacturing the Pd-Ru/Pt catalyst with equivalent precision and performance on a large scale is a complex task. Subsequent investigations should prioritize enhancing the synthesis method to achieve greater cost efficiency and scalability while maintaining the catalyst’s performance.
We conducted a comparative analysis of catalyst combinations with alternative options:
The Pd-Ru/Pt system has exceptional performance in alkaline conditions, surpassing other catalyst combinations like Pd/Pt or Ru/Pt. Although other combinations may have certain advantages, they cannot rival the overall efficiency and stability of the Pd-Ru/Pt catalyst. Pd-Ru/Pt becomes a more appealing choice for applications that require excellent performance in alkaline environments.
However, the cost continues to be a crucial factor to consider. Although Pd-Ru/Pt exhibits superior performance, its higher cost may impact its acceptance, depending on the particular application and budgetary limitations. Mechanism of H2 dissociation and mobility on Pd-Ru@Pt(110) catalyst with free energy landscape.
Environmental and economic advantages:
The enhanced efficacy of the Pd-Ru/Pt catalyst can make a substantial contribution to reducing the carbon footprint associated with hydrogen production and use. The catalyst reduces the energy demands of these processes, hence enhancing the sustainability and environmental friendliness of hydrogen as an energy carrier.
From an economic standpoint, enhancing the efficiency of hydrogen production and utilization can lead to cost reduction and expedite the widespread adoption of hydrogen technology. This has the potential to have extensive and significant impacts, facilitating the shift of the energy industry from fossil fuels to cleaner and more sustainable sources.
In conclusion:
The discovery of a Pd-Ru pair on a Pt surface represents a major advancement in catalysis for hydrogen oxidation and evolution reactions in alkaline media. This catalyst design incorporates the advantageous properties of palladium, ruthenium, and platinum to produce improved efficiency, stability, and durability in demanding conditions.
Although there are remaining obstacles to overcome, particularly regarding cost and scalability, the potential advantages of the Pd-Ru/Pt catalyst are significant. Further research and addressing these challenges could significantly advance hydrogen technology, establishing this catalyst as a fundamental component of the future energy framework.
Frequently Asked Questions:
1). What factors contribute to Pd-Ru’s effectiveness on the Pt surface as a catalyst for the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER) in alkaline media?
Pd and Ru work together to increase hydrogen’s attraction and separation ability, which speeds up the reaction and uses less energy, making it particularly effective in alkaline environments.
2). What is the mechanism by which the combination of Pd and Ru enhances Pt’s efficiency in hydrogen reactions?
Palladium increases the capacity for hydrogen absorption, whereas ruthenium stabilizes the chemical intermediates, hence enhancing the overall catalytic efficiency of platinum.
3). The primary obstacles to utilizing Pd-Ru/Pt catalysts for industrial use are as follows:
The biggest obstacles are the exorbitant costs associated with materials, as well as the complexities of expanding production while maintaining exceptional performance.
4). Are Pd-Ru/Pt catalysts applicable to alternative electrochemical processes?
Pd-Ru/Pt catalysts are very good at catalyzing reactions, which makes them useful for many electrochemical processes, such as oxygen reduction reactions (ORR) and methanol oxidation.
5). What are the potential environmental consequences of using Pd-Ru/Pt catalysts in hydrogen production?
Pd-Ru/Pt catalysts can enhance the efficiency of hydrogen generation and decrease energy consumption. This results in a significant decrease in the carbon footprint of hydrogen, which serves as an energy carrier. Consequently, these catalysts play a crucial role in promoting more sustainable energy solutions.
For more chemistry blogs, visit chemistry Master