Table of Contents
Preface:
Covalent inhibitors targeting cysteine residues are attracting interest in the pharmaceutical industry, presenting possible advancements in drug design. These inhibitors use reactive chemicals, called covalent warheads, that attach permanently to cysteine residues in proteins. This makes them more selective and effective. The development of multifunctional warheads, particularly those that use ketalized unsaturated saccharides, has opened up new opportunities for targeting disease-associated proteins.
This paper examines the novel function of ketalized unsaturated saccharides as covalent weapons that selectively target cysteine residues. We will talk about the problems with current cysteine-targeting methods, how to make ketalized unsaturated saccharides, and how they might be used as multifunctional medicines.
What are the covalent warheads that target cysteine?
Cysteine-targeting covalent warheads are reactive substances that are designed to bind to cysteine residues in proteins more than other parts of the protein. Cysteine, an exceptional amino acid characterized by a reactive thiol (-SH) group, is essential for protein structure and function. Researchers can use this reactivity to create pharmaceuticals that covalently alter cysteine residues in proteins relevant to diseases, ensuring a more enduring and precise therapeutic impact.
Covalent inhibitors, in contrast to conventional reversible inhibitors, establish a permanent link with their target, leading to extended activity. This is particularly advantageous for disorders requiring prolonged suppression of a protein. Scheme 1
Obstacles Associated with Existing Cysteine-Targeting Strategies:
Despite their potential, cysteine-targeting covalent warheads face numerous challenges. A primary concern is the potential for off-target effects, wherein non-cysteine residues or undesirable proteins may also interact, resulting in adverse effects. Protein structures frequently conceal cysteine residues, complicating selective targeting.
A significant problem is attaining the appropriate equilibrium between reactivity and selectivity. Excessively reactive warheads may bind indiscriminately, whereas less reactive ones may inadequately alter cysteine residues.
Unsaturated Carbohydrates in Pharmaceutical Development:
Saccharides, or sugars, are essential in energy metabolism and medication development. Their structural variety and biocompatibility render them exceptional prospects for medicinal chemistry applications. Unsaturated saccharides, characterized by double bonds in their chemical structure, exhibit increased reactivity, rendering them especially attractive for drug creation.
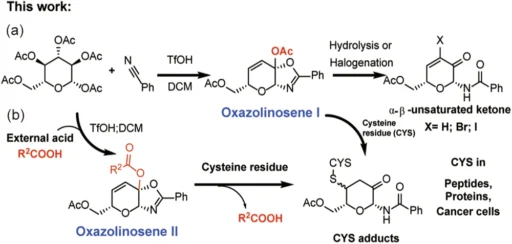
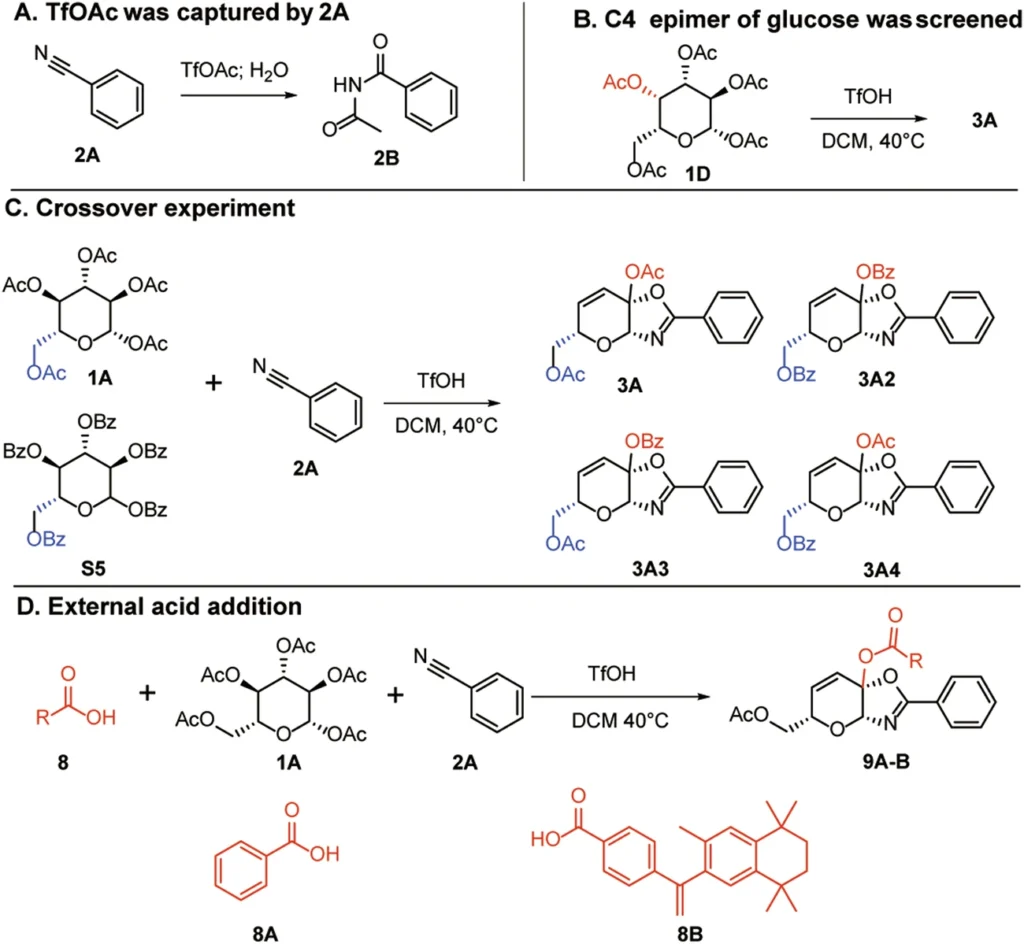
Chemical alteration of these unsaturated sugars can serve as scaffolds for novel medicinal molecules, such as covalent warheads that specifically target cysteine residues. Scheme 2
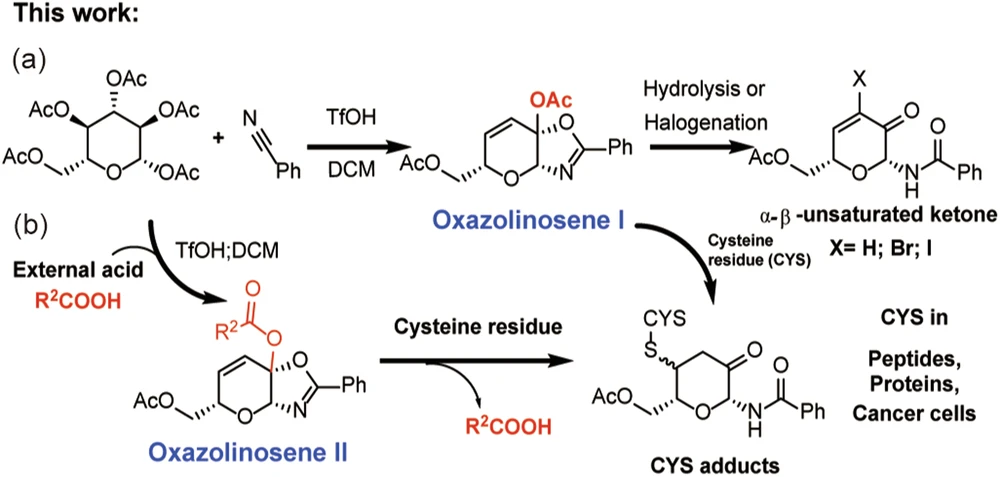
The Concept of Ketalization:
Ketalization is a chemical reaction in which a ketone interacts with an alcohol to form a ketal group. This reaction improves the stability and reactivity of the resultant molecule. Ketalization stabilizes the unsaturated saccharide backbone, enhancing its viability as a therapeutic candidate.
This alteration enables saccharides to endure biological conditions while preserving their capacity for selective reaction with cysteine residues.
Advancement of Ketalized Unsaturated Saccharides:
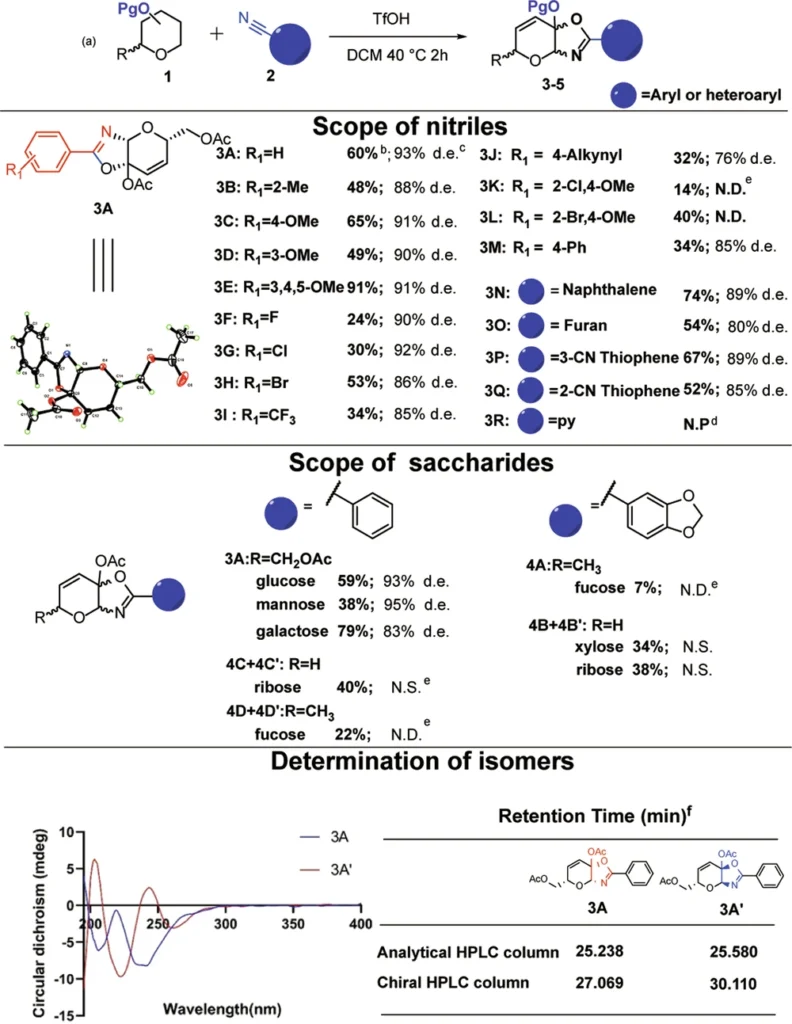
Ketalized unsaturated saccharides constitute a new category of multifunctional weapons. Chemical processes generate them by incorporating both unsaturation (double bonds) and ketal groups into the saccharide framework. This dual modification produces a reactive and stable chemical that specifically targets cysteine residues in proteins.
The process of making these compounds involves carefully picking the saccharide substrates, chemically unsaturated them, and then ketalizing them to make them more stable and reactive. Scheme 3
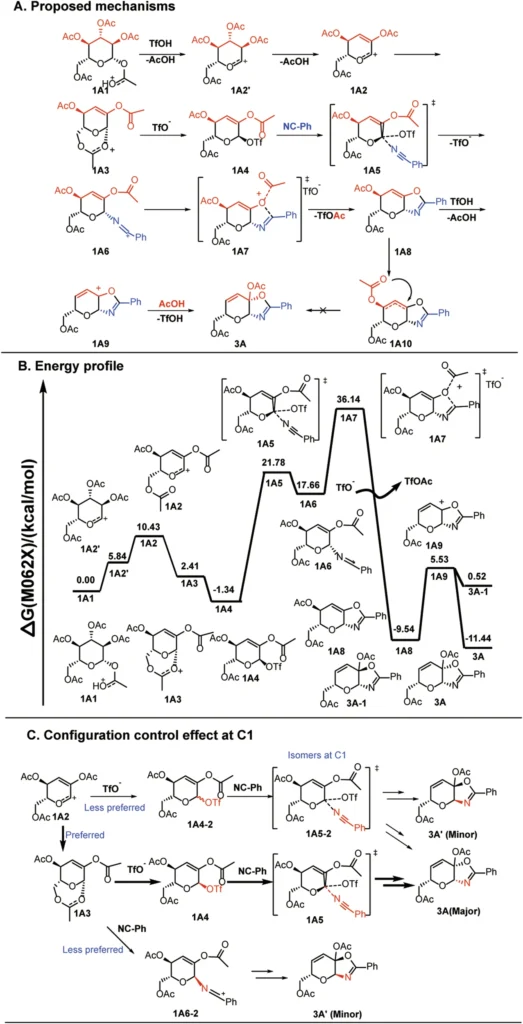
Scheme 4
What is the rationale behind ketalized unsaturated saccharides?
The ketalization of unsaturated saccharides presents numerous benefits compared to conventional cysteine-targeting weapons. Their increased reactivity towards cysteine residues renders them more proficient in covalent inhibition. The ketal group enhances stability, inhibiting early breakdown or reactivity in biological settings.
These saccharides are multifunctional, indicating their capability to execute supplementary biological functions beyond targeting cysteine. For instance, they may possess intrinsic antibacterial or anti-inflammatory activities, hence augmenting their therapeutic potential.
The multifunctionality of ketalized unsaturated saccharides:
The word “multifunctional” denotes the capacity of these warheads to engage in many biological interactions or reactions. In addition to targeting cysteine residues, ketalized unsaturated saccharides may potentially influence other biological pathways, including interactions with other proteins or enzymes.
Their multifunctionality renders them very appealing for therapeutic development, as they may concurrently target numerous disease processes.
Mechanism of Cysteine Targeting:
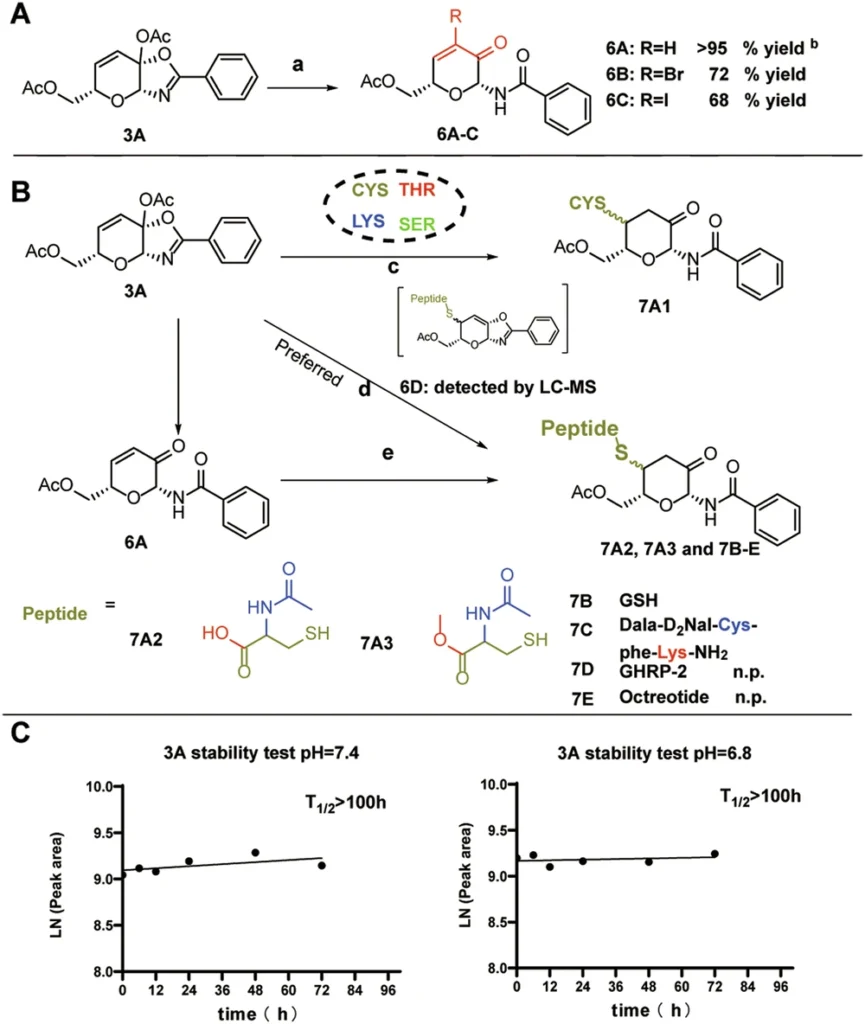
Ketalized unsaturated saccharides covalently link to the thiol group (-SH) of cysteine residues. The unsaturated double bond in the saccharide interacts with the thiol, resulting in a persistent covalent bond that irreversibly alters the protein.
This process obstructs the protein from performing its standard function, effectively suppressing its activity. This covalent alteration is irreversible, providing enduring therapeutic benefits in contrast to reversible inhibitors. Scheme 5
Applications in Pharmaceutical Design:
Ketalized unsaturated saccharides exhibit potential in multiple therapeutic domains, such as oncology, infectious illnesses, and inflammatory disorders. Their capacity to selectively target cysteine residues renders them particularly effective in blocking enzymes or proteins associated with certain disorders.
Many cancer cells, for instance, overexpress proteins containing reactive cysteine residues, rendering them optimal targets for these saccharide-based weapons.
Case Analyses: Initial Development and Investigation
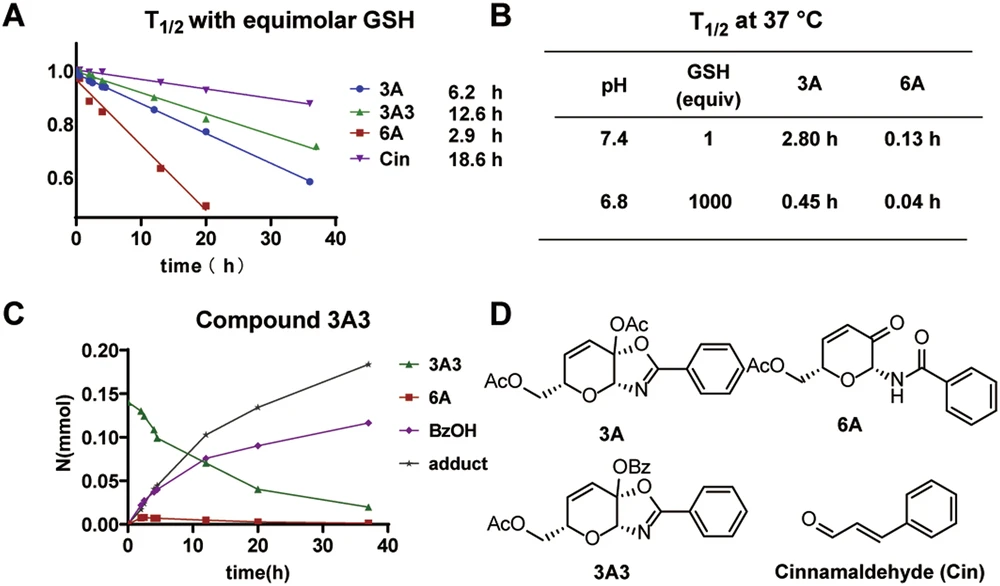
Preliminary investigations into ketalized unsaturated saccharides have demonstrated encouraging outcomes. In preliminary investigations, these warheads exhibited significant selectivity for cysteine residues and diminished off-target effects. A study demonstrated the efficacy of these saccharides in inhibiting cysteine-rich enzymes associated with bacterial infections, indicating promise for antimicrobial therapy. Scheme 6
Benefits Compared to Conventional Warheads:
Ketalized unsaturated saccharides have numerous benefits compared to conventional covalent warheads. Their higher reactivity ensures effective targeting of cysteine residues, and their higher stability lowers the chance of premature degradation. Moreover, their multifunctionality enables involvement in various biological systems, enhancing their therapeutic significance.
Possible adverse effects and hazards:
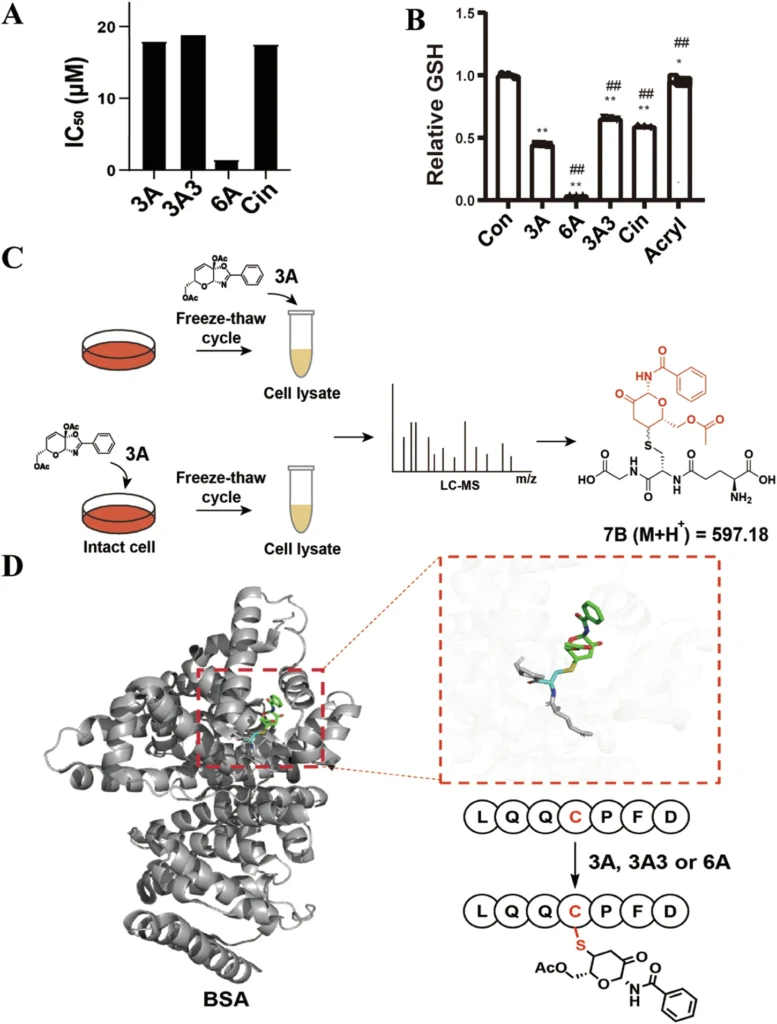
Ketalized unsaturated saccharides, like any medicinal drug, possess inherent hazards. Their elevated reactivity, although advantageous for cysteine targeting, may result in inadvertent interactions with other proteins. Furthermore, prolonged use of covalent inhibitors may cause irreversible alterations to off-target proteins, resulting in harmful effects.
To mitigate these dangers, researchers are concentrating on enhancing the selectivity and diminishing the overall reactivity of these warheads. Scheme 7
Prospective Avenues and Research Prospects:
The future of ketalized unsaturated saccharides involves enhancing their selectivity and investigating novel medicinal applications. Researchers are investigating methods to optimize their reactivity to achieve optimal efficacy with minimal adverse effects. Furthermore, extending its use to additional disease domains, including neurodegenerative disorders, is a promising research direction.
Final Assessment:
Ketalized unsaturated saccharides signify a notable progression in cysteine-targeting covalent inhibitors. Their distinctive amalgamation of heightened reactivity, stability, and multifunctionality renders them a compelling domain of inquiry in pharmaceutical development. As the discipline advances, these warheads have the potential to transform therapies for a wide range of diseases.
Frequently Asked Questions:
1. What are covalent warheads, and what is their significance?
Covalent warheads are reactive compounds that create irreversible interactions with target proteins, providing enduring therapeutic effects in drug design.
2. In what manner do ketalized unsaturated saccharides enhance medication targeting?
These saccharides increase reactivity with cysteine residues while maintaining stability, making them particularly potent cysteine-targeting agents.
3. Are there any hazards linked to the utilization of cysteine-targeting warheads?
Potential hazards encompass off-target consequences and irreversible protein alterations; nonetheless, researchers are endeavoring to enhance selectivity and mitigate side effects.
4. What diseases could benefit from this technology?
Ketalized unsaturated saccharides exhibit potential in the treatment of cancer, infectious illnesses, and inflammatory disorders.
5. What are the subsequent steps for study in this domain?
Future research will concentrate on enhancing the selectivity of these warheads and investigating novel therapeutic uses across many disease domains.
For more chemistry blogs, visit chemistry Master