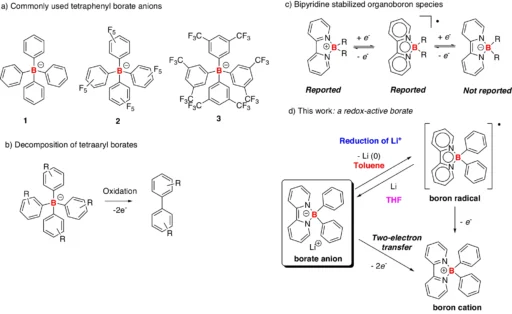
Table of Contents
Lithium-Ion Chemistry: An Overview
Lithium-ion batteries (LIBs) have transformed energy storage and use in contemporary society. Lithium-ion batteries (LIBs) are the preferred technology for efficient energy storage and retrieval, utilized in portable devices, electric vehicles (EVs), and large-scale renewable energy storage systems. Central to these batteries is lithium (Li), one of the lightest and most energy-dense elements in the periodic table. Lithium ions (Li+) are essential for the functionality of these batteries, as they transfer between the anode and cathode during the charging and discharging processes.
The reduction of Li+ is a crucial process that facilitates the efficient operation of lithium-ion batteries (LIBs). This reduction transpires when a lithium-ion acquires an electron, converting it into a neutral lithium atom. The medium in which this reduction occurs can significantly influence the battery’s performance and safety. Borate anions have shown promise for improving the characteristics of lithium-ion batteries.
It is known that boron chemistry produces borate anions, which can form stable complexes with lithium ions. These complexes affect the reduction of Li+, altering the electrochemical characteristics of the system and perhaps enhancing battery performance. This article examines the decrease of Li+ in the presence of borate anions, focusing on the underlying chemistry, its implications for battery technology, and prospective research avenues. Examples and reactivity of tetraaryl borates.
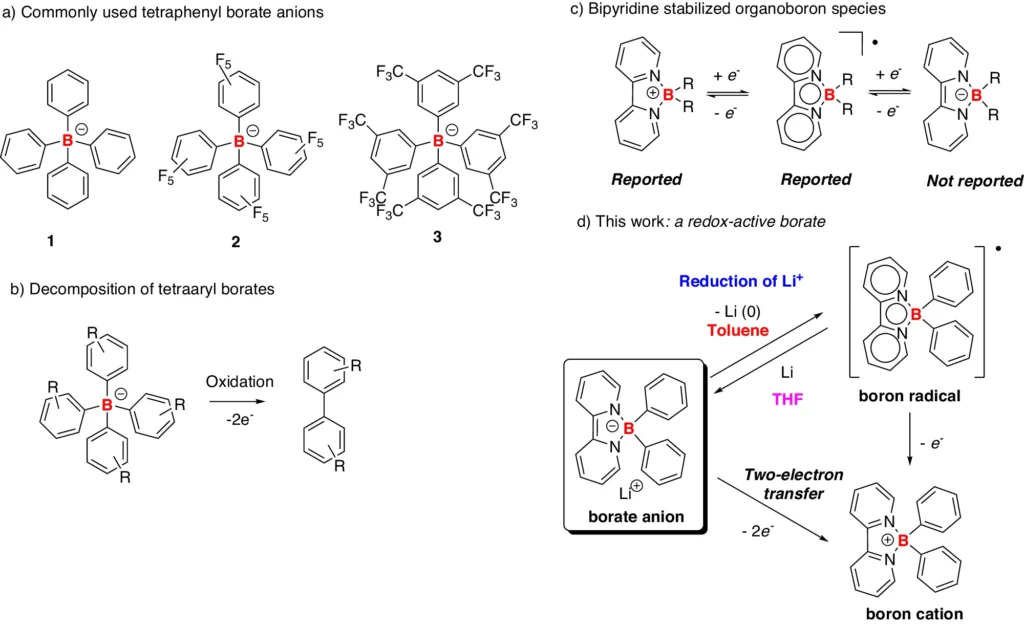
Chemical Characteristics of Lithium and Borate:
An Overview of Lithium:
Lithium, an alkali metal in Group 1, is the lightest and least dense solid element. Its distinctive features render it exceedingly reactive, especially with water and air. In its ionic state, Li+ is stable and diminutive, possessing a high charge density, which renders it optimal for applications in batteries necessitating elevated energy density.
The capacity of lithium to readily lose and gain an electron is what renders it essential for electrochemical energy storage. When lithium ions reduce (gain an electron), they produce lithium metal (Li), which subsequently stores energy for future use. This reaction is essential in the charging cycle of a lithium-ion battery.
An Examination of Borate Anions:
Borate anions (B(OH)₄⁻ and similar species) are generated from boric acid and comprise a core boron atom around oxygen atoms. These anions exhibit diverse shapes contingent upon the chemical environment, including tetrahedral and planar geometries. A fundamental property of borate anions is their capacity to form stable complexes with metal cations, such as lithium ions.
The distinctive characteristics of borate anions render them advantageous in several chemical processes. Borate anions can join with lithium ions in lithium-ion batteries, which changes the reduction potential and keeps lithium species stable in solution.
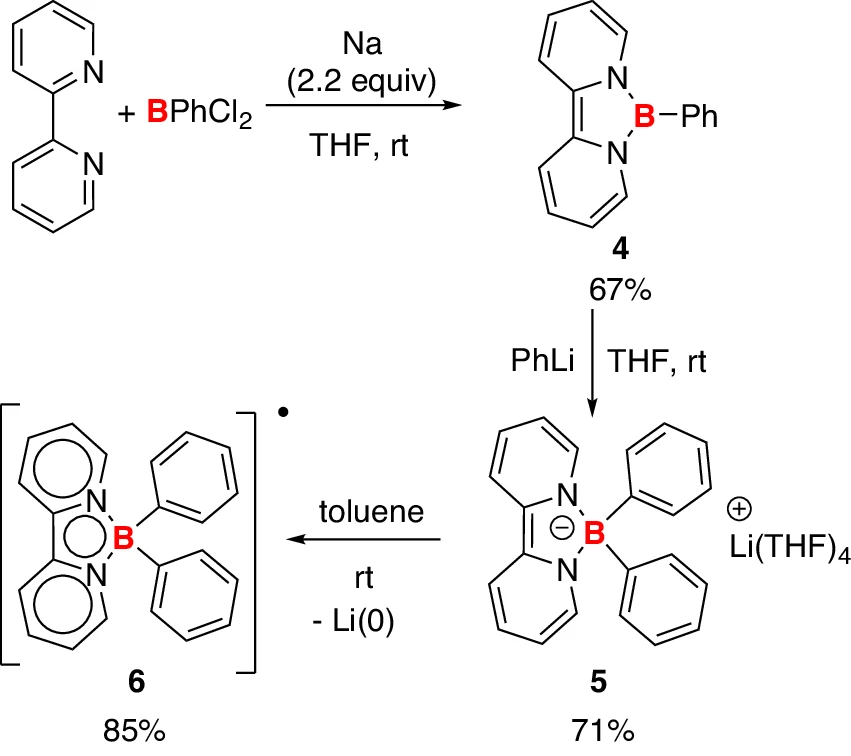
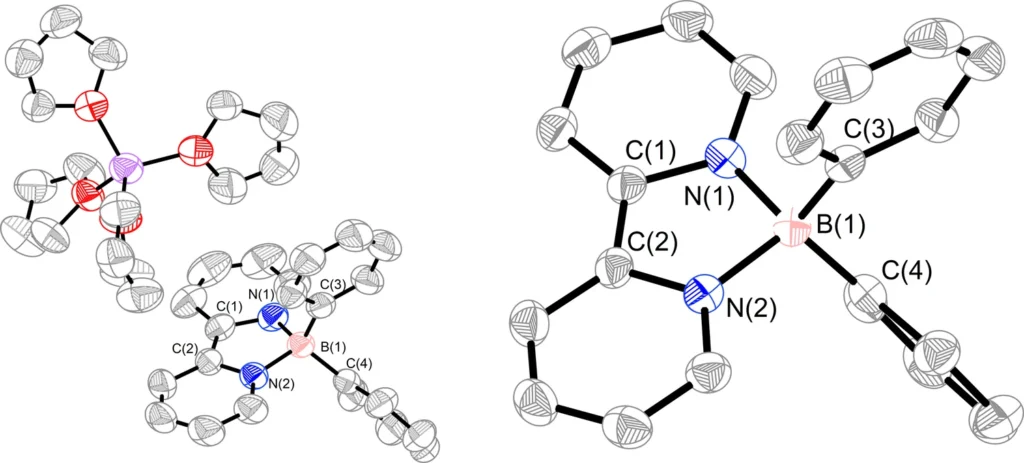
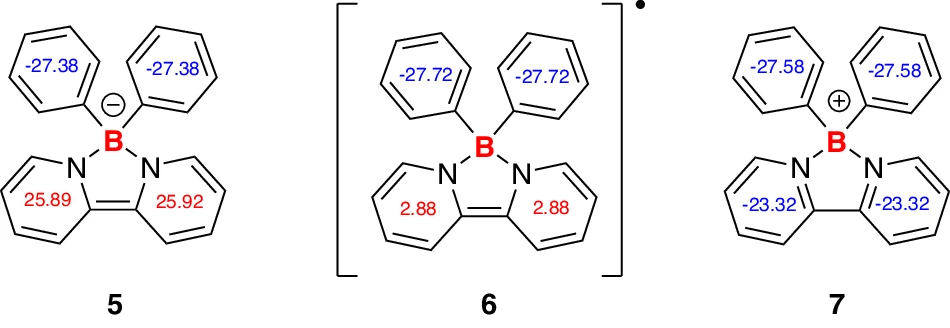
Synthesis of borate anion 5 and boron radical 6. 5 was synthesized from compound 4 and PhLi, and the decomposition of 5 in toluene generated 6 and metallic lithium.
Comprehending the reduction of lithium-ion (Li+):
What constitutes a reduction reaction?
In chemistry, a reduction process entails an atom or ion acquiring electrons. This is a component of a broader category of reactions termed redox (reduction-oxidation) reactions, in which one species undergoes reduction while another experiences oxidation. During the charging cycle of lithium-ion batteries, the reduction of Li+ (gaining an electron) is a crucial step that transforms electrical energy into chemical energy.
Reduction of Lithium-Ion in Batteries:
The decrease in Li+ occurs at the cathode during the charging of a lithium-ion battery. When a lithium-ion receives an electron, it transforms into lithium metal (Li), which stays in the electrode material until the battery discharges. During discharge, the inverse process transpires, wherein lithium atoms relinquish electrons (oxidation), reverting to lithium ions that migrate through the electrolyte to the anode.
This redox reaction is crucial for the functionality of lithium-ion batteries, facilitating the efficient storage and release of electrical energy. During this reduction process, the electrolyte environment has a significant impact on the battery’s efficiency, stability, and safety.
Overview of Borate Anions:
Chemical Structure of Borate Anions:
Borate anions are polyatomic ions composed of boron and oxygen. The most common shape, B(OH)₄⁻, has a boron atom in the middle that is arranged in a tetrahedral shape and has four hydroxyl groups (OH⁻) attached to it. These anions can manifest in multiple forms, such as metaborate (BO₂⁻) and other oligomeric configurations, contingent upon the solution’s pH and concentration.
Borate anions have an unoccupied p orbital on the boron atom, which lets it form strong covalent bonds with oxygen. Numerous industrial applications, such as glass-forming agents and corrosion inhibitors, utilize borate anions for their capacity to create stable structures.
Complexation with Lithium Ions:
One of the most notable characteristics of borate anions is their capacity to form stable complexes with lithium ions. In solution, lithium ions can interact with borate anions, forming coordination complexes that stabilize the lithium-ion. This interaction is critical in electrolyte solutions, as the stability of lithium ions can influence the battery’s overall performance.
Borate anions can change the reduction potential of lithium ions, which makes them a useful part of complex electrolyte mixtures for lithium-ion batteries.
Lithium Ion Reduction Mechanism in Electrochemical Systems:
The electrochemical context of lithium reduction:
In an electrochemical cell, Li+ reduction occurs at the cathode. Lithium ions receive electrons during the charging process, which leads to their reduction to lithium metal. The electrolyte in which this reaction takes place is critical in determining the reduction process’s efficacy. Electrolytes must exhibit stability under the battery’s working conditions and facilitate the efficient movement of lithium ions between the anode and cathode.
Borate-based electrolytes have borate anions that interact with lithium ions. These anions change the reduction potential and keep the lithium-ion stable in solution. This contact is advantageous as it can avert side reactions, including the development of lithium dendrites, which may result in short circuits and battery malfunction.
Sequential Procedure for Lithium Ion Reduction:
Electron Transfer: The reduction of Li+ commences with the transfer of one electron from the external circuit to the lithium-ion. The electron decreases the Li+ ion, transforming it into neutral lithium metal (Li).
Formation of Lithium Metal: When the reduced lithium atom remains in solution or deposits onto the electrode surface, it forms lithium metal. Lithium accumulates on the cathode material in most battery applications, remaining until the battery discharges.
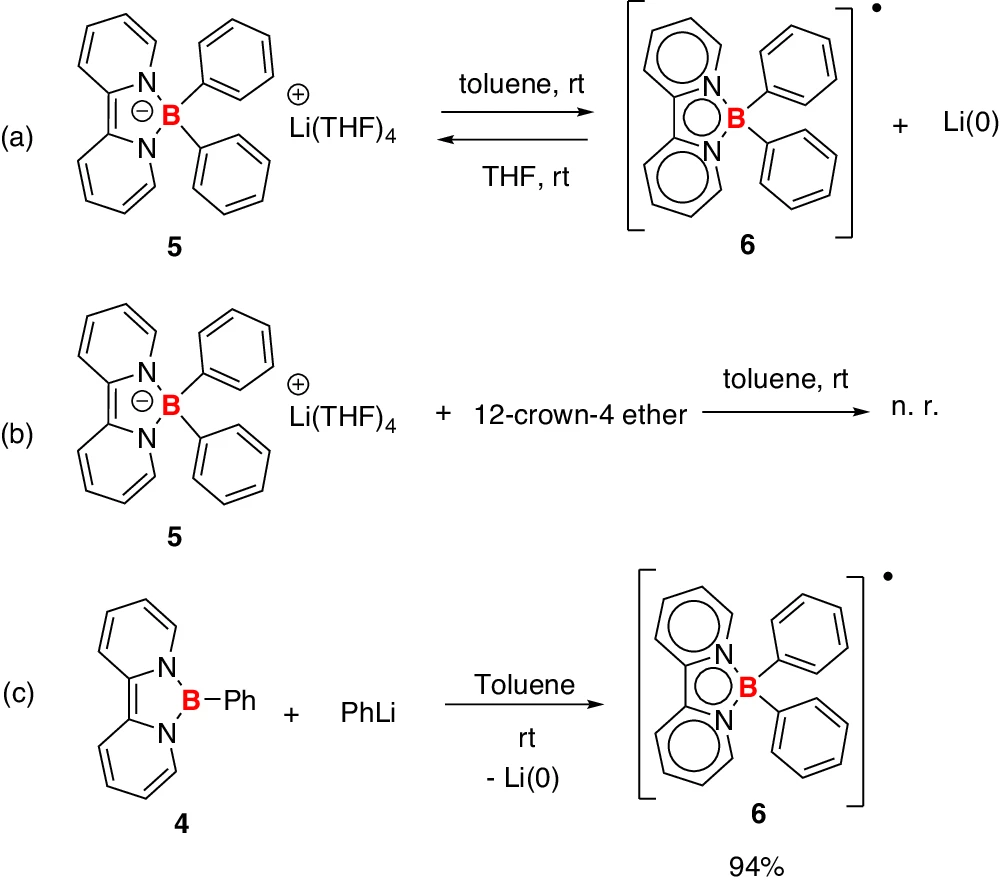
Influence of the Electrolyte: The presence of borate anions in the electrolyte can stabilize the lithium-ion before and after reduction, hence influencing this process. This stabilization is necessary for averting undesirable side effects and guaranteeing the seamless functioning of the battery. Control experiments.
Interactions between Lithium Ions and Borate Anions:
Formation of Lithium Borate Complexes:
When Li+ is present in a solution with borate anions, the two species may combine to form a coordination complex. The borate anion stabilizes the lithium-ion by coordinating with it via the oxygen atoms. This coordination reduces the amount of energy used to reduce Li+, improving the process’s efficiency.
Thermodynamic and Kinetic Factors:
Thermodynamic and kinetic variables govern the production of lithium-borate complexes. From a thermodynamic standpoint, the complex’s stability is dependent on the comparative strengths of the interactions between Li+ and the borate anion against other species in solution. The speed at which complexes form could affect how well the electrochemical cell works overall since faster complex formation could lead to better reduction efficiency.
Effect on Electrochemical Performance:
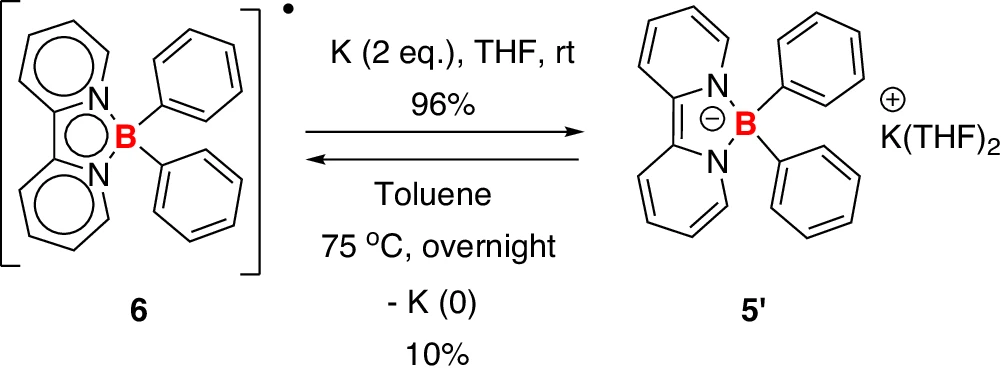
Stable lithium-borate complexes can significantly influence the efficacy of lithium-ion batteries. Borate anions can inhibit side reactions, including lithium dendrite production, by stabilizing lithium ions in solution. This stabilization enhances the safety and efficiency of the battery, especially in high-energy applications where dendrite development frequently occurs. Synthesis of borate anion 5’ and boron radical 6.
Borate’s Effect on Lithium-Ion Reduction Potentials:
Alterations in Redox Potential:
One significant effect of borate anions on the reduction of Li+ is their ability to alter lithium’s redox potential. In a conventional lithium-ion battery, Li+ reduction occurs at a designated voltage about the anode. The presence of borate anions can modify this voltage, either decreasing or increasing it based on the interaction between the lithium-ion and the borate anion.
Empirical Evidence:
Experimental studies indicate that borate anions can alter the reduction potential of lithium ions in solution. Cyclic voltammetry investigations have shown that borate anions can decrease the voltage required for lithium reduction, hence enhancing the process under specific conditions. This alteration in potential enhances the performance optimization of lithium-ion batteries, especially in applications necessitating elevated energy densities.
Electrochemical Properties of Borate Complexes in Batteries:
Stabilizing Lithium Ions in Aqueous Solution:
A major advantage of using borate anions in lithium-ion batteries is their ability to stabilize lithium ions in solution. This stabilization inhibits the development of lithium dendrites, which are needle-like formations that may emerge on the electrode’s surface during charging. Dendrites may penetrate the separator of a battery, resulting in a short circuit and potentially culminating in catastrophic failure.
Borate anions stabilize lithium ions in solution, hence inhibiting dendrite formation and enhancing the battery’s safety and longevity.
Effect on Electrochemical Stability Window:
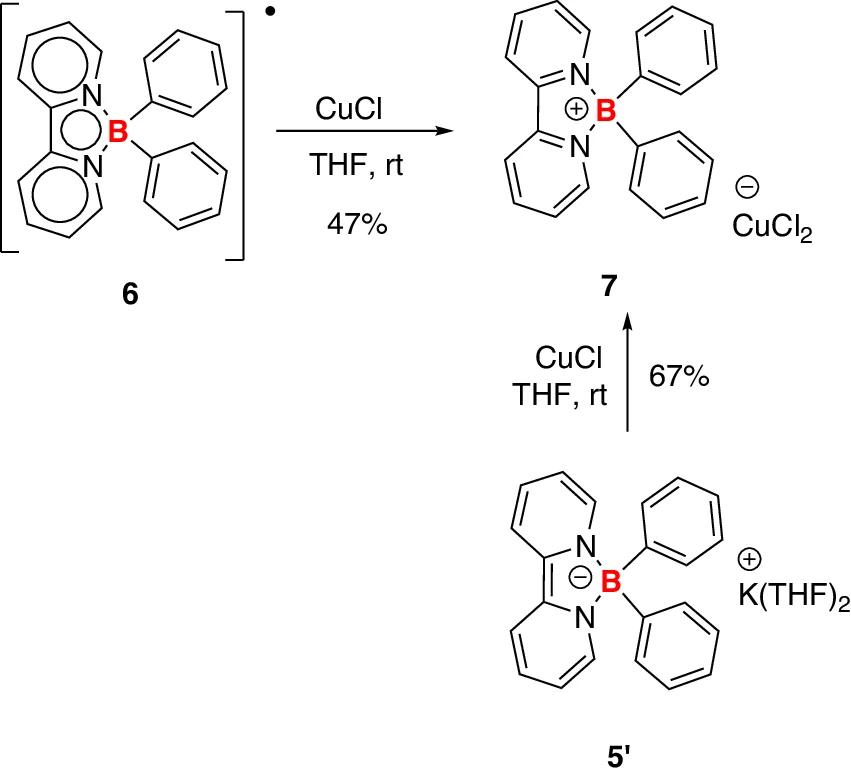
The electrochemical stability window of an electrolyte denotes the voltage range within which the electrode maintains stability and does not undergo decomposition. Borate anions have demonstrated the ability to extend this stability window, enabling the battery to function at elevated voltages without undergoing electrolyte deterioration. The extended stability window is critical in high-energy applications, such as electric vehicles, where elevated operating voltages are required to achieve extended driving ranges. Two different synthetic approaches to compound 7.
Lithium-ion batteries and Borate-Based Electrolytes:
The Rationale for Utilizing Borate in Sophisticated Electrolyte Formulations:
Sophisticated electrolyte compositions for lithium-ion batteries increasingly use borate anions due to their ability to stabilize lithium ions and prevent side reactions. These innovative electrolytes provide numerous benefits compared to conventional electrolyte formulations, such as enhanced safety, increased energy density, and extended cycle life.
Besides their stabilizing attributes, borate anions enhance the thermal stability of the electrolyte, rendering them suitable for high-temperature applications, including electric vehicles and grid storage systems.
Examples of Borate-Based Electrolytes in Commercial Batteries:
Numerous commercial lithium-ion batteries currently utilize borate-based electrolytes to improve performance and safety. Some electric car manufacturers have implemented borate-based electrolytes in their battery packs to enhance thermal stability and avert electrolyte breakdown at elevated voltages. Similarly, grid storage systems that require extended cycle life and high energy density have also benefited from the incorporation of borate anions in their electrolyte compositions.
Investigative Findings on Li+ Reduction Utilizing Borate Anions:
Approaches for Investigating Lithium Reduction:
Researchers have employed multiple experimental methodologies to investigate the decrease of Li+ in the presence of borate anions. Cyclic voltage is a widely employed technique that enables researchers to monitor the real-time reduction and oxidation of lithium ions. This method gives important information about the redox potentials of lithium in different electrolyte conditions and shows how borate anions affect the reduction process.
Researchers have employed additional methodologies, such as electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS), to investigate the interactions between lithium ions and borate anions with enhanced specificity. These techniques yield insights into the stability of lithium-borate complexes and the overall efficacy of the electrolyte inside a battery system.
Principal Discoveries from Empirical Research:
Empirical research has repeatedly demonstrated that borate anions enhance the efficiency and stability of lithium-ion reduction. Cyclic voltammetry tests have shown that borate anions lower the voltage needed for lithium reduction. This makes the process more useful in a wider range of situations.
Electrochemical impedance spectroscopy has also shown that electrolytes based on borate have less resistance to ion transport, which makes the battery work better overall. The reduced resistance results in expedited charging durations and enhanced energy production, rendering borate-based electrolytes especially appealing for high-performance applications.
Utilization of Li+ Reduction in Borate-Based Electrolytes:
Consumer Electronics:
The use of borate-based electrolytes in lithium-ion batteries for consumer electronics is becoming increasingly widespread. Devices like smartphones, laptops, and tablets necessitate batteries that are both energy-dense and safe, as well as durable. Borate anions facilitate these objectives by stabilizing lithium ions in solution, inhibiting side reactions, and prolonging battery lifespan.
Electric Vehicles (EVs):
Electric vehicles exemplify a highly potential application for borate-based electrolytes. The demand for high-energy batteries capable of safe operation at elevated voltages renders borate anions an optimal constituent of electric vehicle batteries. Borate-based electrolytes make EV batteries better by stabilizing lithium ions and expanding the electrochemical stability window. This makes driving ranges longer, charging times faster, and safety better.
Specialized Applications:
Borate-based electrolytes are under investigation for specialized uses, including aerospace and military technologies. These applications necessitate batteries capable of functioning in harsh settings, encompassing elevated temperatures, reduced pressures, and high-radiation environments. Borate anions are ideal for these demanding circumstances because of their robustness and adaptability.
Solid-state and lithium-sulfur batteries:
In addition to their use in conventional lithium-ion batteries, researchers are investigating the use of borate anions in next-generation battery technologies such as solid-state batteries and lithium-sulfur batteries. Solid-state batteries, utilizing a solid electrolyte in lieu of a liquid counterpart, present the possibility of enhanced energy density and superior safety. Borate anions are being investigated as constituents of these solid electrolytes, potentially aiding in the stabilization of lithium ions and enhancing ionic conductivity.
Lithium-sulfur batteries, which possess the potential for superior energy densities compared to lithium-ion batteries, may also gain advantages from borate-based electrolytes. The incorporation of borate anions could alleviate the problems these batteries face, such as inadequate cycle life and the development of lithium dendrites.
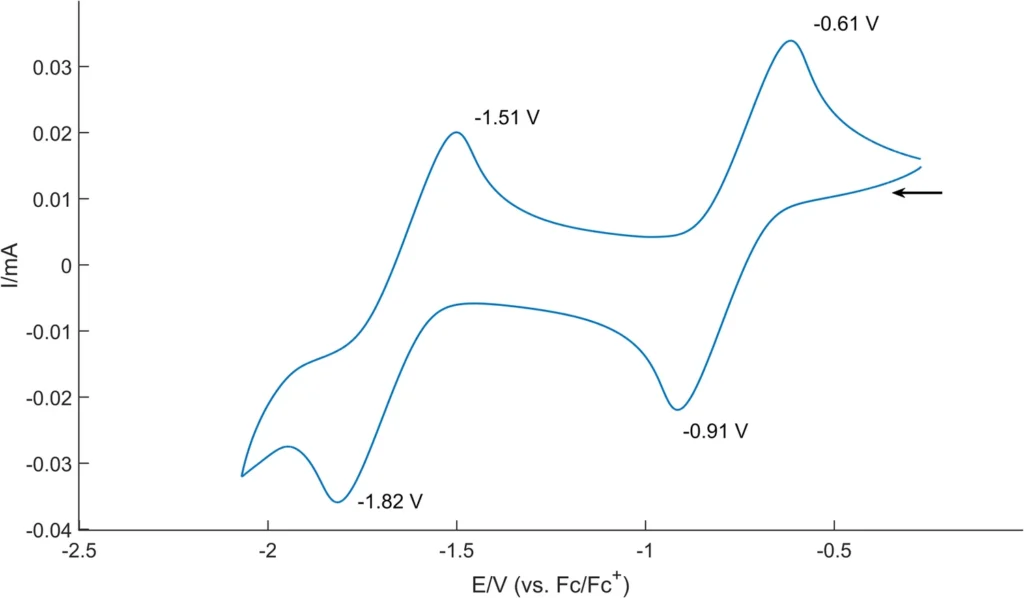
Cyclic voltammograms (CVs) of 6 in THF solution containing 0.1 M [nBu4N][PF6]) at room temperature (scan rate: 100 mV/s). Ferrocene/ferrocenium couple was used as an internal standard.
Lithium Ion and Borate Interactions: Theoretical Models and Simulations
Computational Chemistry’s Role in Battery Design:
Theoretical models and simulations are essential for comprehending the interactions between lithium ions and borate anions. These models assist researchers in forecasting the behavior of lithium and borate under various situations, which is essential for the development of more efficient and stable electrolytes.
Density Functional Theory (DFT) and Molecular Dynamics (MD) Models:
Density functional theory (DFT) and molecular dynamics (MD) simulations are two prevalent computational approaches in this domain. Researchers employ Density Functional Theory (DFT) to determine the electronic structure of lithium-borate complexes, which provides insights into their redox potentials and stability. Researchers can use MD simulations to look at how lithium ions and borate anions move and interact over time. This gives them a dynamic view of the reduction process.
Insights Acquired from Theoretical Frameworks:
Theoretical models have yielded significant insights on the decrease of Li+ in the presence of borate anions. According to DFT calculations, the formation of lithium-borate complexes reduces the energy barrier for Li+ reduction, improving the process’s efficiency. MD simulations have shown that borate anions stop lithium ions from sticking together, which lowers the chance of dendrite formation.
These findings are essential for the development of next-generation electrolytes that provide enhanced performance and safety.
Using Borate in Lithium-Ion Reduction: Obstacles and Constraints
Challenges Associated with Borate Anions in High-Energy Batteries:
Borate anions have numerous benefits for lithium-ion batteries; however, they also pose certain problems, especially in high-energy applications. A primary concern is the propensity for borate anions to break down under extreme circumstances, including elevated temperatures or high voltages. This decomposition may result in the generation of undesirable byproducts, which can impair the battery’s functionality.
Decomposition of Electrolytes:
The degradation of the electrolyte itself is another issue. Borate anions can stabilize lithium ions but may also react with other electrolyte components, resulting in deterioration over time. This deterioration can diminish the battery’s cycle life and elevate the likelihood of failure.
Constraints in Practical Implementations:
Alongside these technological problems, practical constraints also hinder the deployment of borate-based electrolytes in real-world scenarios. The synthesis of borate anions can be expensive, and incorporating these anions into current battery manufacturing techniques may necessitate substantial alterations.
Potential Resolutions:
Notwithstanding these issues, numerous viable solutions exist to address the constraints of borate anions in lithium-ion batteries. One strategy involves integrating borate anions with supplementary electrolyte additions to improve their stability and efficacy. Another strategy involves the creation of novel borate-based materials that exhibit enhanced resistance to degradation under severe conditions.
Recent Developments in Borate-Based Electrolytes:
Novel Materials Integrating Borate Anions:
Recent studies have focused on the development of novel materials that incorporate borate anions into their framework. These materials have enhanced ionic conductivity and stability, rendering them suitable for high-performance lithium-ion batteries. Engineers have engineered borate-based ionic liquids to exhibit exceptional thermal stability and minimal volatility, making them suitable for high-temperature applications.
Enhancements in Electrolyte Stability and Ionic Conductivity:
Recent research mostly concentrates on enhancing the stability and ionic conductivity of borate-based electrolytes. Researchers have created novel formulations that integrate borate anions with additional chemicals, including phosphates or sulfates, to improve their efficacy. The novel electrolytes provide enhanced conductivity and extended cycle life, rendering them optimal for next-generation lithium-ion batteries.
Improvements in Safety and Efficiency:
Another area for improvement is the development of safer and more efficient borate-based electrolytes. Borate anions make lithium-ion batteries safer by stopping lithium dendrites from forming and expanding the electrochemical stability window. These gains are especially significant for applications like electric automobiles, where safety is paramount. NICS(1) values of compounds 5, 6, and 7.
Comparison of Borate Anions with Alternative Anionic Complexes:
Substitutes for Borate in Lithium-Ion Electrolytes:
Although borate anions provide numerous benefits for lithium-ion batteries, alternative anionic complexes are equally applicable in electrolyte formulations. Common alternatives comprise phosphate anions, carbonate anions, and sulfate anions. Each of these anions possesses distinct advantages and disadvantages, contingent upon the individual use.
The benefits and drawbacks of using Borate differ from those of Phosphate, Carbonate, and other alternatives:
In comparison to phosphate or carbonate anions, borate ions exhibit enhanced stability and the capacity to form more robust complexes with lithium ions. Nonetheless, borate anions may incur higher production costs and necessitate more intricate manufacturing procedures. Phosphate anions are comparatively more economical and demonstrate satisfactory performance in several applications; nonetheless, they may lack the stability offered by borate.
Reasons for Borate’s Enhanced Efficacy in Specific Applications:
Borate anions exhibit notable efficacy in high-energy and high-voltage applications, where stability and safety are paramount. Their capacity to stabilize lithium ions and inhibit side reactions renders them optimal for application in electric vehicle batteries and other high-performance systems.
The impact of Borate-Based Electrolytes on the environment and economy is significant:
Sustainability of Borate Production and Utilization:
People typically view the generation of borate anions as more sustainable than the synthesis of certain other electrolyte constituents, like fluorinated chemicals. We source borate from boron, a reasonably plentiful element, and its manufacture has a lower environmental impact than certain other chemicals used in battery manufacturing.
Ecological Advantages Compared to Traditional Electrolytes:
Borate-based electrolytes provide numerous ecological advantages over traditional electrolytes. They exhibit reduced toxicity and enhanced recyclability, rendering them a more sustainable alternative for future battery technologies. Moreover, their capacity to inhibit side reactions and enhance the safety of lithium-ion batteries diminishes the likelihood of environmental contamination resulting from battery failure.
Financial Implications for Extensive Manufacturing:
Although borate-based electrolytes have numerous benefits, their manufacturing may be costlier than conventional electrolyte formulations. The increased expense arises from the complexity of synthesizing borate anions and incorporating them into current battery production methodologies. However, we anticipate that as research advances and new techniques emerge, the cost of borate-based electrolytes will decrease, making them more competitive with conventional alternatives.
Recycling and Disposal of Borate-Based Electrolytes:
A primary benefit of borate-based electrolytes is their recyclability. We can efficiently extract and use borate anions, thereby mitigating the environmental consequences of lithium-ion battery disposal. Because of their recyclability, borate-based electrolytes are a more sustainable choice for future energy storage.
Final Assessment:
The diminution of Li+ within a borate anion framework signifies a viable strategy for enhancing the performance, safety, and sustainability of lithium-ion batteries. When lithium ions are stabilized, side reactions are stopped, and the electrochemical stability window is expanded, borate anions are very helpful. These characteristics render them suitable for high-energy applications, including electric vehicles and grid storage systems.
Despite the challenges associated with borate anions, such as probable disintegration under harsh conditions and elevated production costs, recent advancements in materials science and electrolyte formulation have mitigated these concerns. Ongoing research indicates that borate-based electrolytes will significantly contribute to the advancement of next-generation lithium-ion batteries, enhancing performance, safety, and sustainability.
Frequently Asked Questions:
1). What renders borate anions appropriate for lithium-ion batteries?
Borate anions stabilize lithium ions in solution, inhibit dendrite development, and enhance electrolyte stability, resulting in improved battery performance and safety.
2). What is the mechanism by which Li+ is reduced in borate electrolytes?
Getting electrons at the cathode reduces lithium ions, and the presence of borate anions keeps the process stable, which lowers the chance of side reactions.
3). What challenges are associated with the use of borate in batteries?
Borate anions may disintegrate under harsh conditions, and their synthesis is costlier than conventional electrolyte components.
4). Are borate-based electrolytes ecologically sustainable?
Indeed, borate-based electrolytes have reduced toxicity, enhanced recyclability, and diminished environmental effects relative to traditional electrolytes.
5). What are some of the potential applications of borate anions in energy storage?
Borate anions are under investigation for applications in electric vehicles, solid-state batteries, lithium-sulfur batteries, and other high-energy technologies owing to their stability and efficiency.
For more chemistry blogs, visit chemistry Master