Table of Contents
Introduction of Liver Cancer:
Liver cancer remains one of the most aggressive forms of cancer, with high mortality rates and limited treatment options. Hepatocellular carcinoma (HCC), the most common type of liver cancer, is difficult to treat due to the organ’s vital role in metabolism, detoxification, and its unique immune environment. Despite advances in cancer therapies, such as chemotherapy, radiation, and immunotherapy, liver cancer patients often face recurrence and drug resistance. One major problem in Liver Cancer is that many therapies fail to effectively deliver drugs to the liver without causing significant side effects elsewhere in the body.
Targeted therapy has emerged as a critical approach for improving the effectiveness of cancer treatments while minimizing the collateral damage to healthy tissues. An exciting notion in targeted liver cancer therapy is to exploit naturally occurring mechanisms, such as the transport networks used by the liver. Among these mechanisms, bile acid-binding proteins (BABPs) offer a promising pathway to transport therapeutic agents specifically to liver tissues. Researchers can enhance the transport of an anti-cancer medicine to the site of greatest need by conjugating it with a molecule that the liver naturally recognizes and processes.
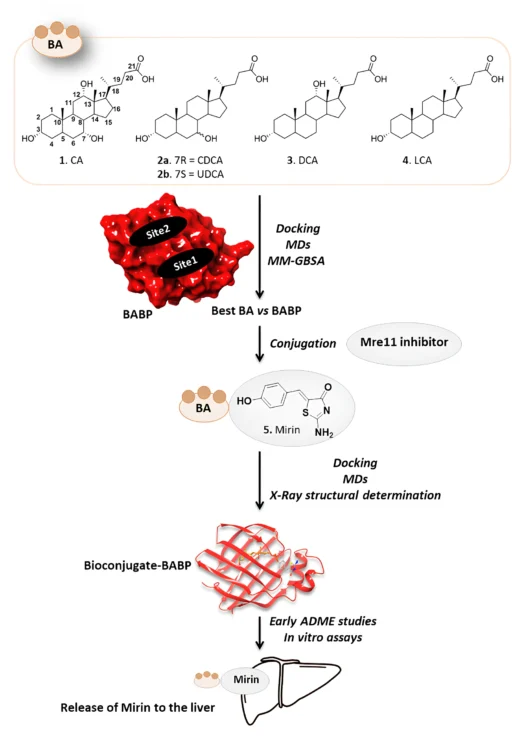
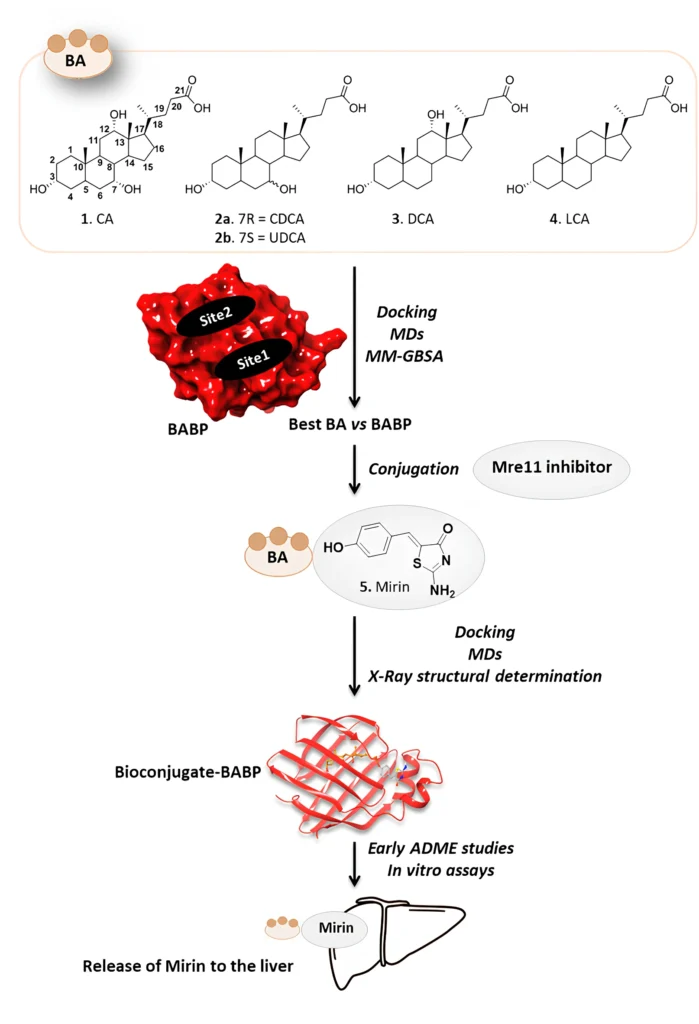
Putting mirin, a strong drug inhibitor, together with cholic acid, a bile acid that the liver naturally absorbs, creates a new way to treat this condition. This article talks about using bile acid binding proteins as transporters for a cholic acid/Mirin bioconjugate. It focuses on the mechanism, the pros and cons, and what the future holds for liver cancer treatment.
Bile Acid Binding Proteins: An Overview
Definition and Function of Bile Acid Binding Proteins (BABPs):
Bile acid binding proteins (BABPs) are a class of intracellular proteins important for binding bile acids and aiding their transport inside cells, especially in the liver and intestine. The liver produces these proteins, stores them in the gallbladder, and releases them into the intestine to aid in digestion and fat absorption, all while maintaining the homeostasis of bile acids.
The liver synthesizes bile acids from cholesterol and conjugates them with either glycine or taurine to improve their solubility. The enterohepatic circulation transports most bile acids back to the liver after the intestine performs its digestive functions. To efficiently transport bile acids within the liver cells and re-secrete them into bile, BABPs play a crucial role in managing this complex recycling process.
The importance of BABPs in liver biology and drug delivery of Liver Cancer patients:
BABPs are highly expressed in the liver due to their critical role in bile acid metabolism, making them potential targets for drug delivery systems. By exploiting BABPs, scientists can develop drug conjugates that mimic bile acids, allowing the drugs to “hitch a ride” with these proteins to reach liver cells. This targeted approach can greatly enhance the precision of drug delivery to liver tissues, which is particularly advantageous in liver cancer therapy, where traditional chemotherapy often affects healthy tissues and causes severe side effects.
Cholic Acid: Composition and Role in Treatment of Liver Cancer
Summary of Cholic Acid:
Cholic acid is a principal bile acid synthesized in the liver from cholesterol. There is a steroid backbone in the chemical structure, and hydroxyl groups make it more amphipathic, which means it has both hydrophilic and hydrophobic regions. Because cholic acid is amphipathic, it makes it easier for fats to be broken down into smaller droplets in the intestine, which helps enzymes do their job better.
Researchers extensively studied cholic acid for its role in digestion and potential applications in medication delivery methods. Because liver cells can naturally take in and break down bile acids like cholic acid, some researchers think that adding medicines to cholic acid might help get these medicines to the liver more quickly.
Cholic Acid’s Potential for Drug Conjugation:
The liver’s intrinsic affinity for cholic acid makes it an ideal target for conjugating with medicinal medicines. Drugs containing cholic acid can reach the liver directly through the natural bile acid transport pathways. This selective targeting reduces the likelihood of drug exposure to other organs, thereby minimizing systemic toxicity. In cancer therapy, where high doses of drugs are often required, this approach can significantly improve patient outcomes by increasing drug efficacy at the tumor site while reducing adverse side effects.
Cholic acid can also improve the solubility of hydrophobic drugs, further enhancing their bioavailability and making it easier for the body to absorb and utilize them. Researchers can exploit the liver’s bile acid uptake mechanisms by conjugating a drug to cholic acid, which enhances the drug’s delivery efficiency to liver cells, including cancerous cells.
Mirin is a pharmacological agent:
Introduction to Mirin:
Mirin, a pharmacological agent, is known for its ability to inhibit the ataxia telangiectasia mutated (ATM) kinase, a crucial enzyme involved in DNA repair. ATM plays a vital role in the cellular response to DNA damage, activating repair pathways when double-strand breaks develop. In cancer cells, this DNA repair pathway allows the cells to live and grow despite the genetic damage that would typically trigger cell death. By blocking ATM, Mirin affects the DNA repair process, allowing Liver Cancer cells to accumulate genetic damage, ultimately leading to their demise.
Mirin’s Mechanism of Action in Cancer Therapy:
Cancer cells, particularly those in rapidly developing tumors like liver cancer, are heavily reliant on DNA repair systems to survive the continuous genetic damage that happens during cell division. By suppressing ATM activity, Mirin stops cancer cells from repairing DNA damage produced by both natural cellular processes and exogenous stimuli, such as radiation or chemotherapy. This inhibition puts the cancer cells into a condition of crisis, where they can no longer maintain their genetic integrity and finally suffer apoptosis (programmed cell death).
Mirin’s non-specific dispersion throughout the body is the issue with its use in cancer therapy. Like many anti-cancer drugs, Mirin can damage healthy cells as well as cancer cells, leading to potential adverse effects. This is where the idea of conjugating Mirin with a liver-targeting molecule, such as cholic acid, becomes particularly advantageous. By constructing a cholic acid/mirin bioconjugate, researchers can ensure preferential transport of mirin to liver cancer cells, enabling it to exert its effects without harming healthy organs.
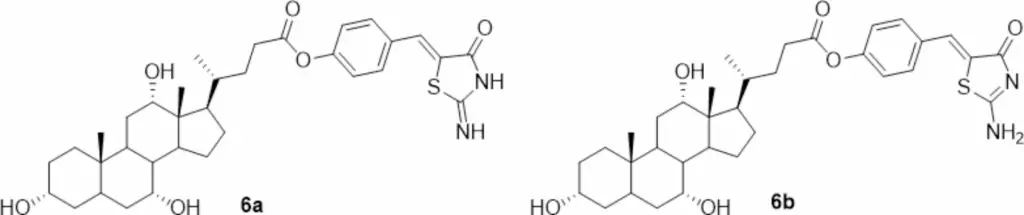
2D structure of iminothiazolidin-4-one tautomer 6a and aminothiazolidin-4-one tautomer 6b of CA-M11.
Bioconjugates in Cancer Therapy:
What Are Bioconjugates?
Bioconjugates are special molecules made by chemically joining a medicine that works on the body with a carrier molecule, like a peptide, protein, or small organic molecule, to help the drug get to where it needs to go more quickly and effectively. The carrier component of the bioconjugate helps direct the medicine to specific tissues or cells, while the active drug portion retains its therapeutic activity. Researchers have extensively studied bioconjugates in cancer therapy to enhance drug delivery precision, boost treatment efficacy, and minimize side effects.
Benefits of Bioconjugates in Targeted Therapy:
A primary benefit of bioconjugates is their capacity to selectively target particular cells or tissues, thereby reducing the exposure of non-targeted cells to the drug’s toxic effects. In cancer treatment, selective targeting is crucial, as traditional chemotherapy agents frequently destroy both malignant and healthy cells, resulting in severe side effects such as nausea, fatigue, and immune suppression.
Bioconjugates can improve pharmaceuticals’ solubility, stability, and bioavailability, hence increasing their efficacy upon reaching the target site. By attaching a drug to a molecule that specific cell receptors recognize and move, bioconjugates can help a drug concentrate in particular tissues, such as tumors. This stops the drug from spreading throughout the body. This method enhances therapeutic outcomes while permitting reduced drug dosages, thereby minimizing the risk of side effects.
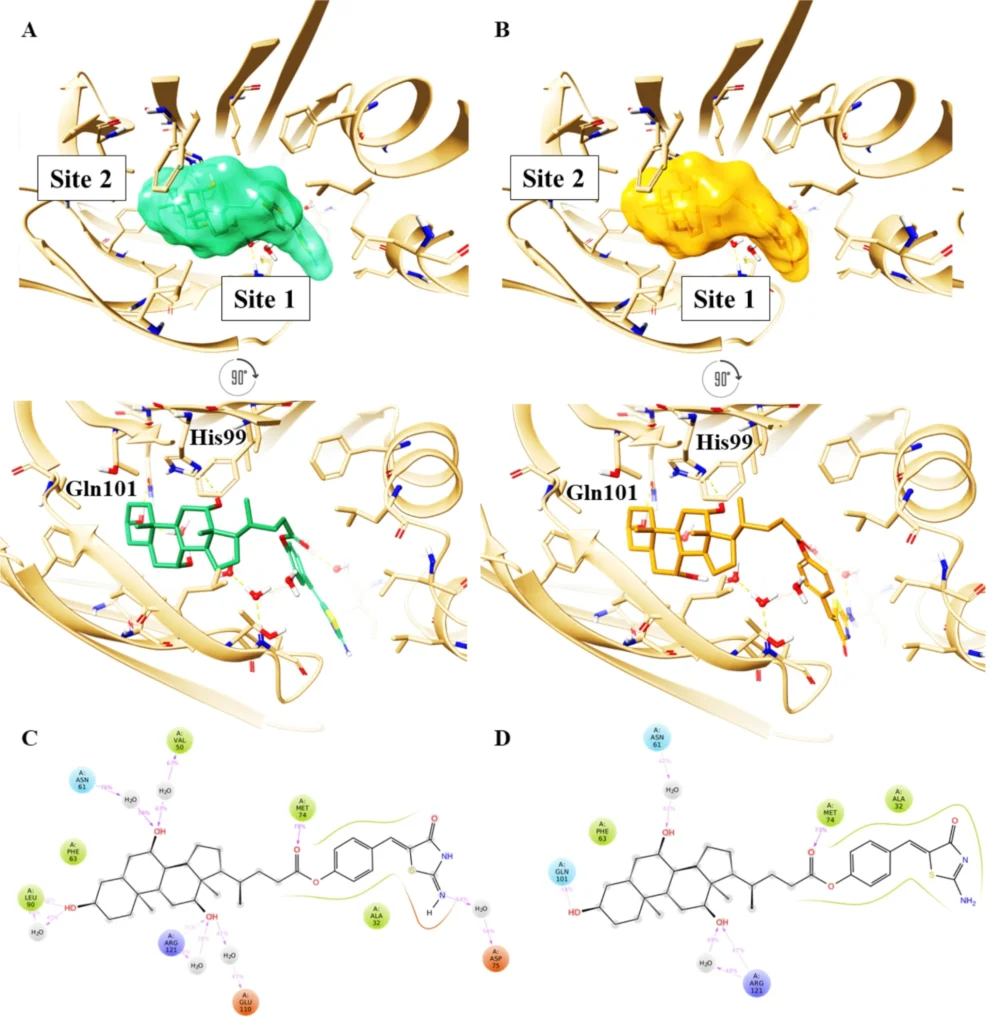
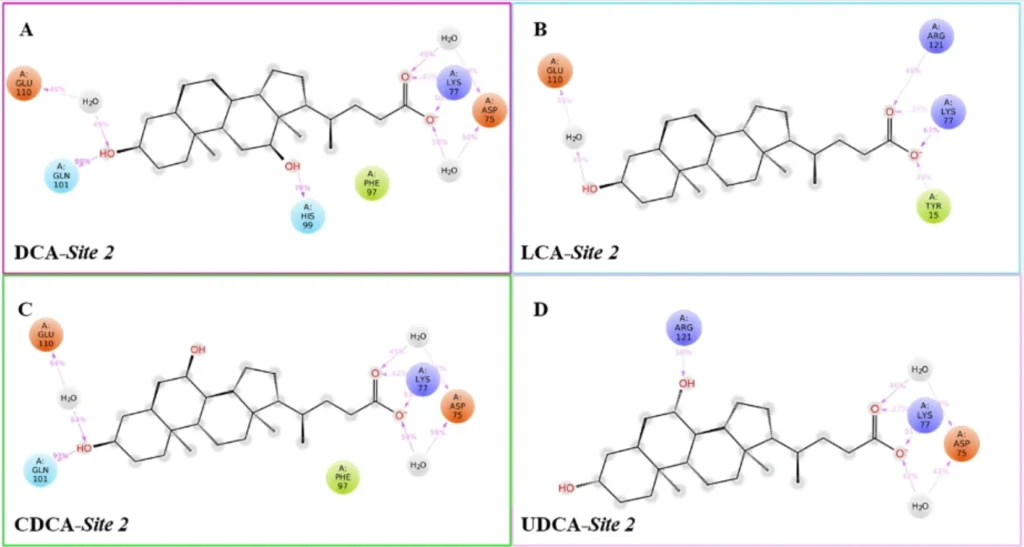
Top and front view of the best docking pose of (A) keto-imino (green carbon sticks) and (B) keto-amino tautomers (orange carbon sticks) of CA-M11 in complex with cL-BABP. The protein is reported as a crème cartoon, with the residues involved in pivotal contacts shown as carbon sticks. The H-bonds are interactions are indicated as yellow dashed lines. Schematic representation of ligand-protein interactions of (C) keto-imino and (D) keto-amino tautomers of CA-M11 into cL-BABP. Interactions occurring in more than 30% of the MDs are reported.
The Cholic Acid/Mirin Bioconjugate Concept:
Fundamental Principles of Bioconjugate Design:
The cholic acid/Mirin bioconjugate design combines cholic acid’s ability to target the liver with Mirin’s ability to stop DNA repair. The objective is to utilize cholic acid as a “Trojan horse” to deliver Mirin into hepatic cells, specifically those that have undergone malignant transformation. The bioconjugate would liberate Mirin upon entering the liver cells, thereby interfering with cancer cells’ DNA repair mechanisms and facilitating their demise.
To ensure the stability of both molecules during bloodstream circulation and enable the release of mirin upon reaching the liver, we must meticulously engineer the chemical conjugation of cholic acid and mirin. Generally, a linker molecule connects the two components and undergoes cleavage in the acidic liver environment or by specialized enzymes present in liver cells. The linker selection is critical, as it must possess sufficient stability to avert premature drug release while being responsive enough to guarantee timely release at the target location.
Advantages of Employing Cholic Acid for Mirin Delivery:
The principal advantage of using cholic acid for Mirin delivery is that the medication is better targeted to the liver. Liver cells inherently absorb bile acids, such as cholic acid, as a function of fat metabolism. By conjugating Mirin with cholic acid, researchers can leverage this natural transport mechanism to guarantee that the drug attains elevated concentrations in the liver. This targeted administration diminishes the exposure of Mirin to other organs, thereby reducing the risk of systemic toxicity.
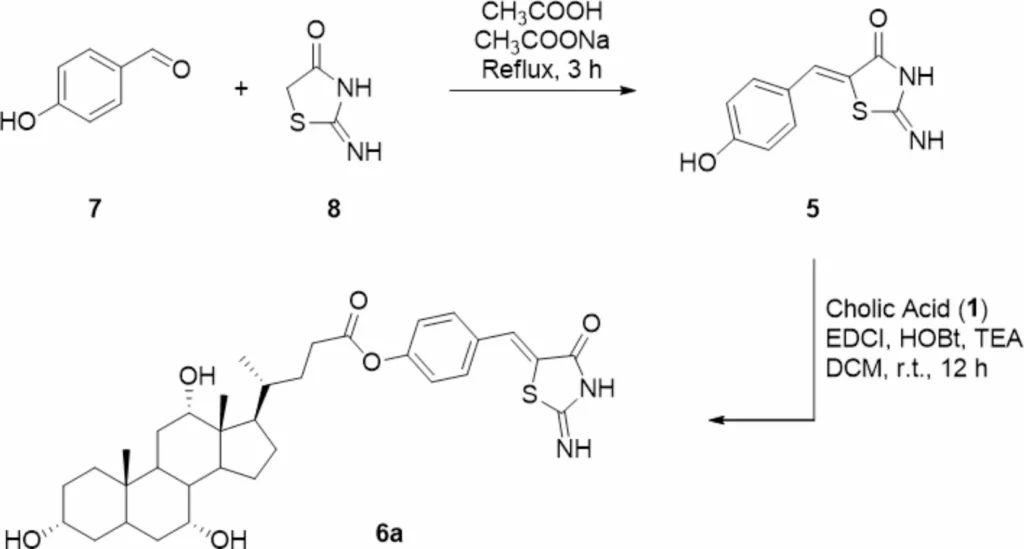
Another benefit is that cholic acid can improve Mirin’s solubility and bioavailability, leading to greater absorption and utilization by the body. This is particularly beneficial for drugs like Mirin, which may have restricted solubility in water and inefficient absorption when delivered in their unbound state. Scheme 1 Synthesis of Mirin (5) and Cholic acid-Mirin bioconjugate CA-M11 (6a). Reagents and conditions are specified in the scheme.
Mechanism of Action: Utilizing Bile Acid Binding Proteins
BABPs’ mechanism of bile acid transport:
BABPs attach to bile acids in hepatocytes and make it easier for them to move through the cell’s cytoplasm to different parts, such as the endoplasmic reticulum and mitochondria. These proteins are crucial for the enterohepatic circulation of bile acids, facilitating their effective recycling between the liver and intestine.
Bile acid binding proteins (BABPs) can bind a bile acid conjugate, such as the cholic acid/Mirin bioconjugate, upon entering the liver, thereby facilitating its transport within the hepatocyte. This intrinsic transport mechanism facilitates the bioconjugate accumulation in hepatic cells, enabling enzymatic processing that cleaves the linker between cholic acid and mirin, thereby releasing the drug in a regulated fashion.
Drug Binding, Transport, and Release Processes at Hepatic Sites:
Binding: Following injection, the cholic acid/Mirin bioconjugate traverses the bloodstream and arrives at the liver. Liver cells exhibit particular receptors that identify and attach to bile acids, including the cholic acid component of the bioconjugate. This binding facilitates the bioconjugate’s uptake into liver cells.
Transport: Once inside the liver cell, BABPs bind to the cholic acid portion of the conjugate and transport it through the cytoplasm. The transport process directs the bioconjugate to the appropriate cellular compartments, like the endoplasmic reticulum or lysosomes, where enzymes are present to process the conjugate.
Release: The liver’s acidic environment, or enzymatic cleavage, breaks the linker between cholic acid and Mirin, releasing the active drug into the liver cell. Once released, Mirin can exert its therapeutic effects by inhibiting DNA repair pathways, leading to the accumulation of genetic damage in cancer cells and triggering their death.
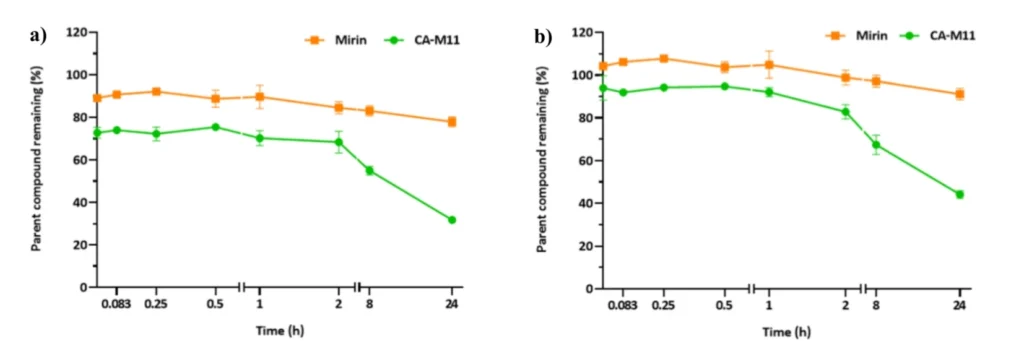
Stability profiles of compounds CA-M11 (red), and Mirin (blue) were obtained in plasma (a) and DMEM 10% FBS (b). The values are reported as mean ± SD on n = 3 experiments run in triplicate. The ability of CA-M11 and Mirin to resist the metabolizing action of human liver microsomes was also investigated.
Pharmacokinetics of the Cholic Acid/Mirin Bioconjugate:
How the Bioconjugate Circulates and Reaches Liver Cells:
The cholic acid/Mirin bioconjugate’s pharmacokinetics describe how it is absorbed, distributed, broken down, and flushed out of the body. After administration, the bioconjugate is absorbed by the bloodstream and circulated until it reaches the liver. Bile acid transporters on liver cells’ surfaces recognize the cholic acid portion of the conjugate, allowing these cells to selectively take up the bioconjugate.
Absorption, Distribution, Metabolism, and Excretion (ADME):
Absorption: The bioconjugate is designed to be absorbed efficiently after oral or intravenous administration. The cholic acid component helps facilitate this absorption, as bile acids are naturally absorbed in the intestine and transported to the liver through the portal vein.
Distribution: The affinity of cholic acid for liver cells primarily distributes the bioconjugate to the liver once it enters the bloodstream. This targeted distribution ensures that the majority of the bioconjugate accumulates in the liver rather than in other tissues.
Metabolism: Within the liver, enzymes degrade the bioconjugate by severing the linker connecting cholic acid and mirin. The medication only activates in the liver because of the regulated release of Mirin, allowing it to specifically target cancer cells.
Excretion: The body ultimately eliminates both cholic acid and mirin through bile or the kidneys, following the effects of mirin. We engineer the bioconjugate to diminish the accumulation of toxic metabolites, thereby lowering the risk of chronic side effects.
Improving Bioavailability via BABPs:
The use of BABPs as carriers enhances the bioavailability of the cholic acid/Mirin bioconjugate by facilitating direct delivery to hepatic cells. This targeted delivery diminishes the necessity for elevated drug dosages, as a greater proportion of the drug arrives at its designated site of action. Furthermore, by protecting the bioconjugate from premature degradation in the bloodstream, BABPs facilitate increased drug availability for uptake by hepatic cells.
Benefits of BABPs as Pharmaceutical Carriers:
Intrinsic Abundance in Hepatic Tissues:
A key advantage of using BABPs as drug transporters is their inherent abundance in hepatic tissues. These proteins, found in large quantities in the liver, efficiently transport bile acid-based drug conjugates, obviating the need for foreign proteins or synthetic delivery methods. This reduces the risk of immunological responses and other issues arising from the introduction of innovative drug delivery systems into the body.
Targeted Affinity for Hepatic Cells:
BABPs demonstrate significant selectivity for bile acids, predominantly metabolized by hepatic cells. BABPs’ specificity makes them ideal for hepatic drug delivery, ensuring that liver cells absorb the majority of the drug conjugate rather than dispersing it throughout the body. This focused delivery is especially vital in liver cancer treatment, as it is essential to concentrate the medication in the liver to enhance its therapeutic efficacy.
Minimized off-target effects relative to conventional therapies:
Conventional chemotherapy agents frequently exhibit considerable off-target effects, harming healthy cells alongside cancerous ones. Utilizing BABPs to administer a cholic acid/mirin bioconjugate enables researchers to mitigate the risk of off-target effects. Researchers engineer the bioconjugate for preferential uptake by hepatic cells, thereby reducing its exposure to other tissues and organs. This focused strategy diminishes the likelihood of adverse effects, enhancing the treatment’s tolerability for patients.
Targeting Hepatocellular Carcinoma with Cholic Acid/Mirin Bioconjugate:
Hepatocytes’ Uptake of Bile Acid and Bioconjugate Mechanisms:
Liver cells possess specialized transporters on their surface that identify and adhere to bile acids. NTCP and OATPs are transporters that let bile acids from the bloodstream get into liver cells. Bile acids either sequester in the bile upon entering the hepatocytes or undergo metabolization to aid digestion.
Researchers can use bile acid transporters to help get medicines directly to liver cells by attaching a drug like Mirin to cholic acid. Similar to natural bile acids, liver cells absorb the bioconjugate, ensuring efficient drug delivery to the liver.
Cancerous cells vs. healthy cells: selective targeting
One of the best things about using a cholic acid/mirin bioconjugate is that it can target only cancerous liver cells and leave healthy cells alone. Liver cancer cells, particularly those in hepatocellular carcinoma, commonly demonstrate altered bile acid metabolism and increased bile acid absorption. This means that cancer cells may take up the bioconjugate more readily than healthy cells, allowing for more effective targeting of the tumor.
In addition, the regulated release of Mirin in the liver guarantees that the medicine is concentrated in the location where it is needed the most, further boosting its capacity to target cancer cells while limiting damage to surrounding healthy organs.
Potential for Minimizing Systemic Toxicity:
Systemic toxicity is a key concern in cancer therapy, as many anti-cancer medications damage not only the tumor but also healthy tissues throughout the body. Researchers can significantly reduce systemic toxicity by primarily administering the medicine to the liver via a cholic acid/Mirin bioconjugate. This focused approach allows for lower drug dosages, minimizing the likelihood of adverse effects such as nausea, exhaustion, and immunological suppression.
Therapeutic efficacy in liver cancer:
How the Bioconjugate Enhances Mirin’s Anti-Cancer Effects:
The cholic acid/mirin bioconjugate makes Mirin’s anti-cancer effects stronger by sending the medicine directly to the liver, where it can stop cancer cells from fixing their DNA. Mirin interrupts these processes, causing cancer cells to accumulate genetic damage, ultimately leading to their demise. The bioconjugate’s targeted distribution ensures that there is more Mirin in the liver, making it more effective against liver cancer.
Preclinical Studies: Efficacy in Liver Cancer Models
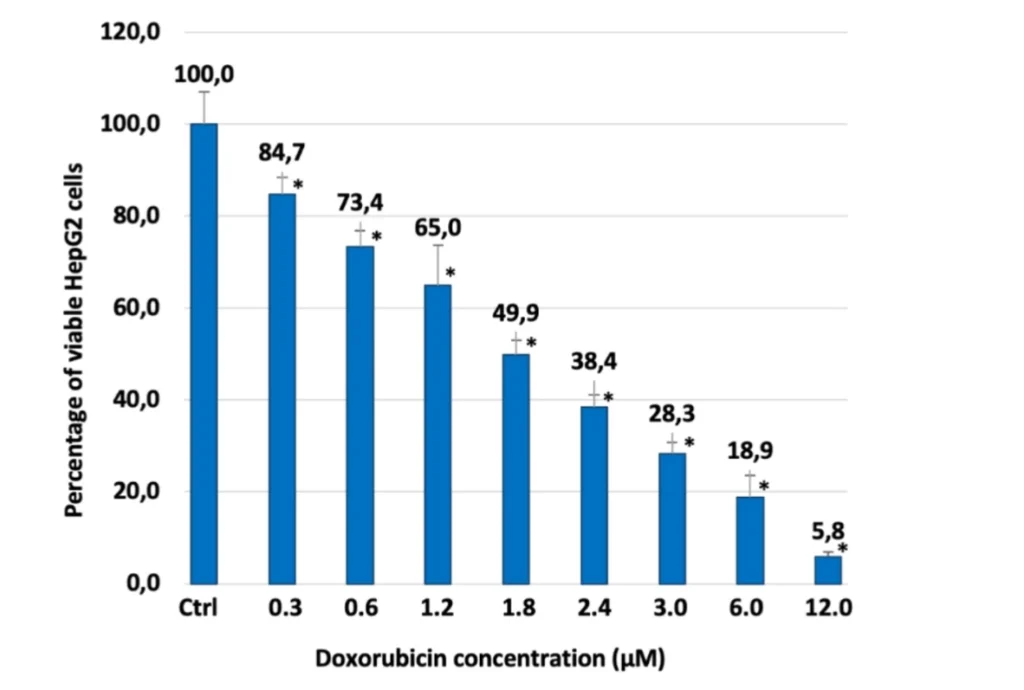
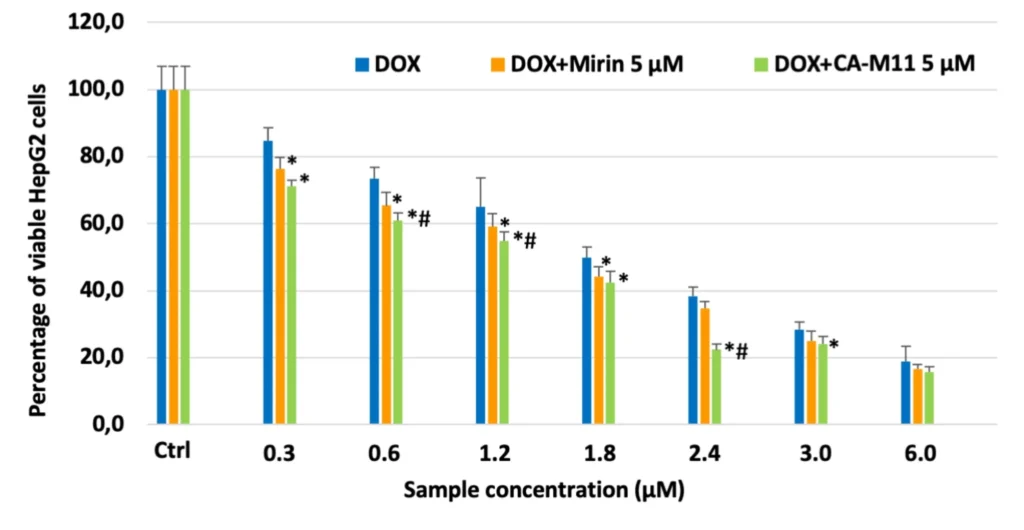
Preclinical investigations of cholic acid-based bioconjugates have shown encouraging outcomes in liver cancer models. In liver cancer patients, animal tests have demonstrated that the cholic acid/Mirin bioconjugate inhibits tumor growth and enhances survival rates. These results demonstrate that the bioconjugate is successful in delivering Mirin to liver cancer cells, where it can exercise its therapeutic effects.
Even though more research needs to be done on humans to confirm these results, the preclinical data strongly support the use of cholic acid/Mirin bioconjugates in liver cancer therapy.
Future Directions in Clinical Applications:
The future of cholic acid/mirin bioconjugates in liver cancer therapy is promising. Clinical trials will be necessary as research advances to ascertain the bioconjugate’s safety and efficacy in human patients. If successful, this approach could become a standard treatment for liver cancer, offering a more targeted and less toxic alternative to traditional chemotherapy.
In addition, researchers are exploring the possibility of using cholic acid-based bioconjugates to deliver other anti-cancer drugs to the liver. This approach could expand to treat a variety of liver diseases, including other forms of cancer and metabolic disorders, by tailoring the conjugate to different drugs.
Challenges and Considerations:
Potential Hurdles in Developing This Therapy:
Although the cholic acid/mirin bioconjugate holds significant potential, its development must address a number of challenges. Maintaining the bioconjugate’s stability within the bloodstream is a primary challenge. If the conjugate degrades too rapidly, it may release the drug prior to reaching the liver, diminishing its efficacy and heightening the risk of adverse consequences.
Another problem involves optimizing the linker between cholic acid and mirin. The linker must exhibit sufficient stability to avert premature drug release while being responsive to facilitate timely drug release in the liver. Researchers are investigating various linkers to achieve an optimal equilibrium between stability and responsiveness.
Drug resistance and degradation challenges:
Similar to any cancer treatment, there is a possibility that liver cancer cells may develop resistance to the cholic acid/Mirin bioconjugate. Cancer cells can modify their mechanisms to diminish bile acid absorption or enhance their capacity to metabolize the drug compound. Researchers are looking into these mechanisms of resistance to come up with ways to stop them. For example, they could use combination therapies or change the bioconjugate to get around resistance.
Ensure the bioconjugate’s stability and efficacy:
Ensuring the stability and efficacy of the bioconjugate is essential for its success as a treatment for liver cancer. Researchers are attempting to refine the chemical composition of the bioconjugate to ensure its stability during bloodstream circulation while facilitating the precise release of the medicine at the appropriate moment and location. This necessitates a meticulous equilibrium between stability and reactivity, alongside continuous evaluation in preclinical and clinical trials.
Safety and Toxicology:
Toxicological Assessment of Cholic Acid with Mirin:
Cholic acid and mirin exhibit tolerable toxicity profiles when utilized separately. However, we must meticulously examine the synergistic effects of the two molecules in a bioconjugate to ensure the absence of unforeseen adverse effects. Toxicology profiling is an important part of developing the cholic acid/Mirin bioconjugate because it helps find safe dose levels and any risks that might come with the therapy.
Ensuring secure pharmacological doses and minimizing adverse effects is crucial:
One of the key goals of using a cholic acid/mirin bioconjugate is to reduce side effects by selectively delivering the medicine to the liver. However, it is still critical to carefully control the bioconjugate dosage to ensure that it does not cause injury to healthy liver cells or other tissues. To determine the optimal dosage levels and monitor patients for potential negative effects, clinical trials will be necessary.
Long-term safety concerns for liver cancer patients:
Long-term safety is another crucial factor in the development of the cholic acid/Mirin bioconjugate. While short-term trials may reveal promising outcomes, it is necessary to analyze the long-term effects of the medication, particularly in patients with liver cancer who may require ongoing treatment. Researchers will need to follow individuals for any potential long-term concerns, such as liver damage or other issues.
Future of Bioconjugates in Cancer Therapy:
Emerging Trends in Bioconjugate Development:
Bioconjugates are fast emerging as a new frontier in cancer therapy, with multiple active studies evaluating their potential to deliver medications more effectively and selectively. We expect the creation of novel bioconjugates for a wide spectrum of malignancies, including liver cancer, as research continues. The cholic acid/mirin bioconjugate exemplifies the application of this technique in enhancing cancer treatment efficacy.
Expanding the focus from liver cancer to other cancer types:
Specifically designed for liver cancer, the cholic acid/Mirin bioconjugate applies the principle of targeting specific tissues to various types of cancer. Researchers are investigating the application of bioconjugates for drug delivery to tumors in many organs, including the lungs, pancreas, and brain. By customizing the carrier molecule for specific target tissues, bioconjugates possess the capacity to transform cancer treatment across many tumor types.
The Function of Personalized Medicine in Bioconjugate Therapy:
With the advancement of bioconjugate technology, personalized medicine will assume a progressively significant role in cancer treatment. Physicians can choose the most suitable bioconjugate therapy for a patient’s tumor by examining its genetic and molecular attributes. This tailored methodology will guarantee that patients with Liver Cancer have the most efficacious treatment for their particular cancer type, enhancing their survival prospects and minimizing the likelihood of adverse effects.
Final Assessment:
A promising new way to treat liver cancer is to use bile acid binding proteins to help a cholic acid/Mirin bioconjugate move through the body. This technique utilizes the Liver Cancer, the liver’s own bile acid transport pathways to preferentially target cancer cells, enhancing therapeutic efficacy and reducing side effects. A bioconjugate of cholic acid and mirin has shown a lot of promise in preclinical tests. With more research, it could become an important part of treating liver cancer.
Despite ongoing hurdles in enhancing the stability, efficacy, and safety of bioconjugates, the prospects for bioconjugate-based therapeutics are promising. This new strategy may provide more targeted, effective, and personalized cancer treatments, extending beyond liver cancer to encompass various malignancies as research progresses.
Frequently Asked Questions:
1). What distinguishes bile acid-binding proteins as unique drug transporters for Liver Cancer?
BABPs are prevalent in the liver and have a strong affinity for bile acids, rendering them suitable for Liver Cancer of liver-targeted treatments.
2). In what way does cholic acid help to target liver cancer cells?
Hepatocytes inherently absorb cholic acid, which facilitates the targeted delivery of conjugated pharmaceuticals to hepatic tissue.
3). What is the significance of using Mirin in cancer treatment?
Mirin obstructs DNA repair processes in neoplastic cells, facilitating apoptosis and diminishing tumor proliferation.
4). What are the potential adverse effects of utilizing bioconjugates?
Despite their ability to mitigate systemic toxicity, bioconjugates may still exhibit negative effects, particularly if they release the conjugate prematurely into the bloodstream.
5). What are the prospective trajectories for bioconjugate therapy in hepatocellular carcinoma?
Future research may concentrate on enhancing bioconjugate stability and broadening their application to other malignancies and personalized treatment strategies.
For more chemistry blogs, visit chemistry Master