Table of Contents
Overview of Meta-C-H Sulfonylation:
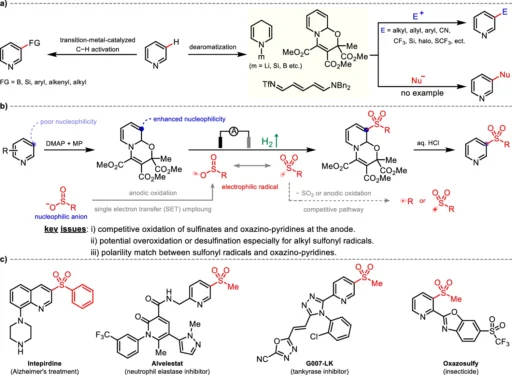
The introduction of C-H functionalization has revolutionized the area of organic synthesis, empowering chemists to change basic hydrocarbons into intricate compounds precisely. Pharmaceuticals, agrochemicals, and material science commonly use pyridines, making them particularly significant among the many heterocycles. An especially fascinating alteration is the sulfonylation of pyridines, which introduces sulfonyl groups that enhance the molecule’s chemical characteristics. Conventional approaches to this transition can be burdensome, frequently necessitating severe conditions or intricate reagents. But now there is a new way to do it that is better for the environment and works just as well: electrochemical Meta-C-H Sulfonylation using nucleophilic sulfinates.
Comprehension The process involves functionalizing C-H bonds through meta-selective reactions:
Meta-C-H Sulfonylation changes the hydrogen atom in the meta-position about a functional group on an aromatic ring in a particular way. Although substituents have a directing effect that makes it easier to target the ortho and para positions, the meta-circumstance presents considerable hurdles. Attaining site selectivity in this specific situation is a remarkable accomplishment of contemporary synthetic chemistry, and it has significant importance when it comes to altering intricate structures such as pyridines. Background and motivation of pyridine meta-C–H functionalization.
Pyridines’ Significance in Organic Synthesis:
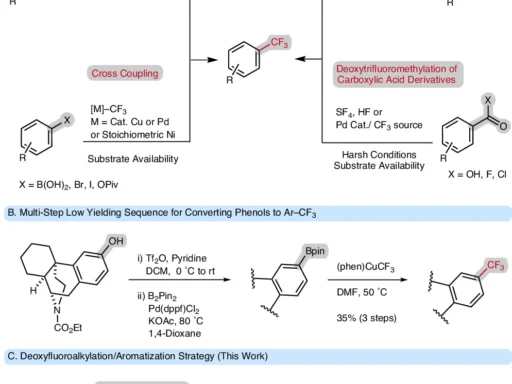
For example, pyridine has a six-member aromatic ring with one nitrogen atom. They are the building blocks of many chemicals that are biologically active. Because of their diverse chemistry and capacity to function as ligands, they are extremely useful in the field of drug design and development. In the past, process techniques such as halogenation or metal-catalyzed cross-coupling processes have achieved the modification of the pyridine ring. ures frequently lack specificity or necessitate numerous stages, underscoring the need for a more direct procedure.
Meta-C-H Sulfonylation is a crucial reaction in organic chemistry:
Sulfonylation is the process of adding a sulfonyl group (-SO2R) to a molecule, which has a substantial effect on its physical and chemical characteristics. Sulfonyl groups have a vital role in medicinal chemistry due to their potential to promote molecular stability, improve solubility, and regulate biological activity. Conventional sulfurylation methods usually employ sulfonyl chlorides or anhydrides. Although these methods are successful, they often demand strict reaction conditions or produce byproducts that require complicated purification procedures. Reaction scope of electrochemical meta-sulfonation of pyridines.
Utilizing Electrochemical Methods for Organic Synthesis:
Electrochemistry has become more popular in organic synthesis as an effective method for promoting chemical reactions. Electrochemical technologies provide a clean alternative to standard reagent-based processes by utilizing an electric current to stimulate redox reactions. These reactions frequently take place in less severe conditions, resulting in fewer secondary products, which makes them appealing for both academic and industrial purposes. A wide range of chemical processes, including oxidations, reductions, and coupling reactions, have effectively used electrochemical techniques, demonstrating their adaptability.
An overview of electrochemical Meta-C-H Sulfonylation:
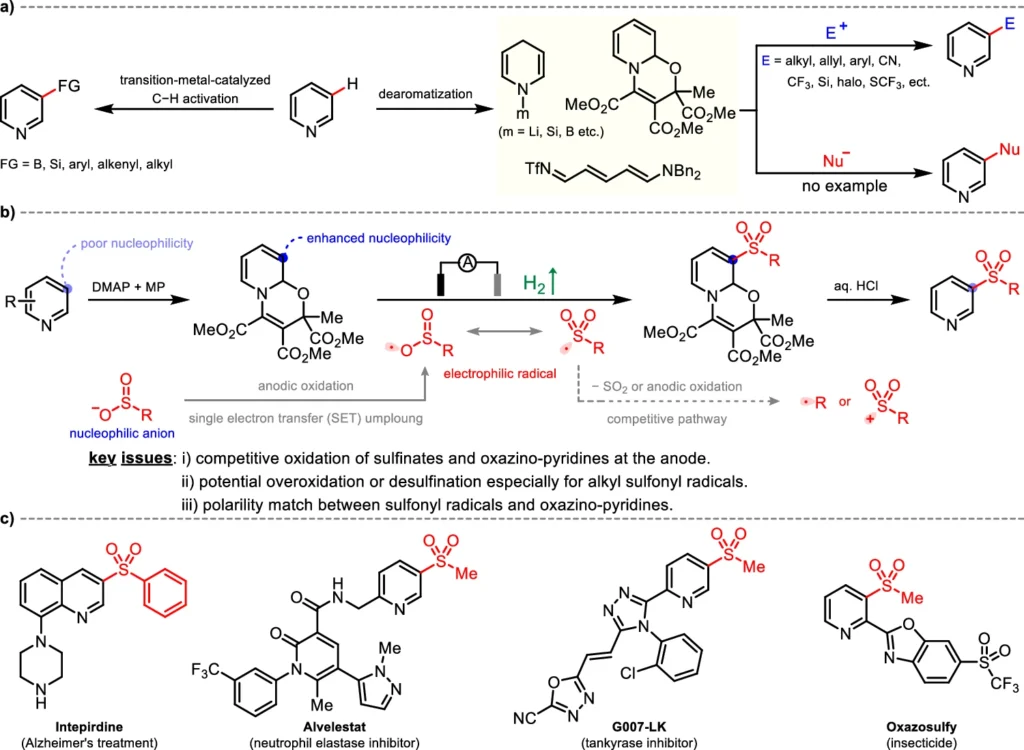
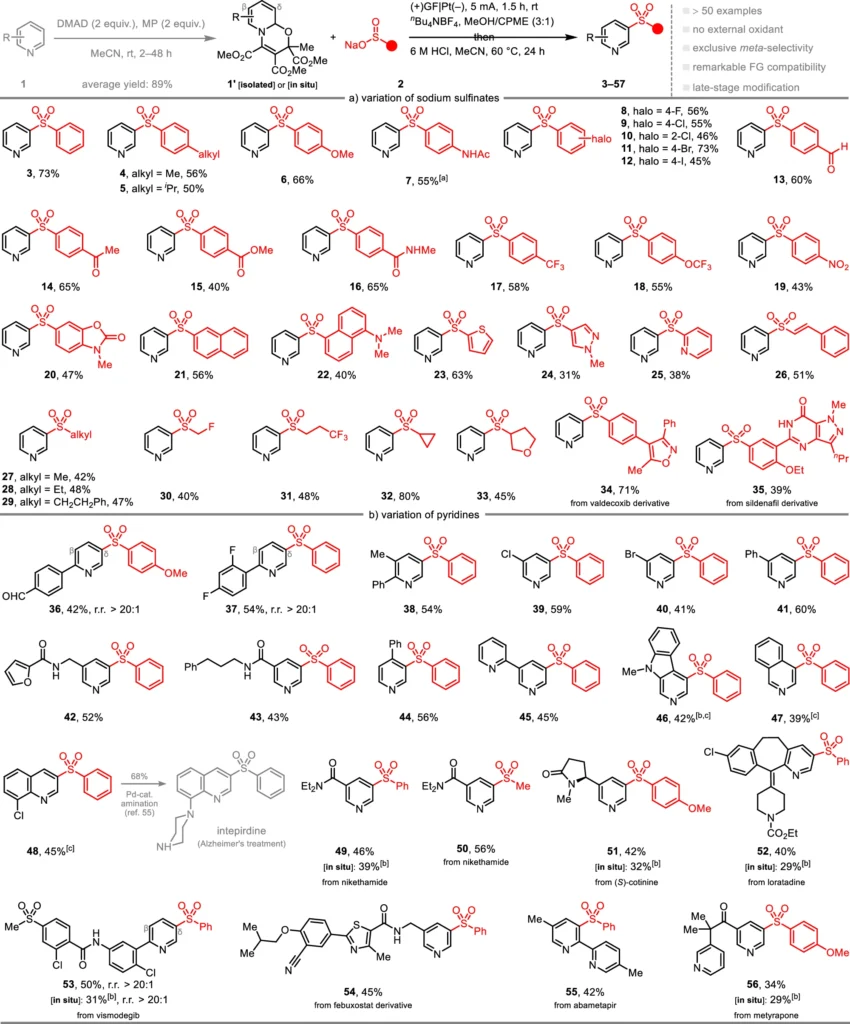
A big step forward in the field of C-H functionalization is the electrochemical Meta-C-H Sulfonylation of pyridines. This reaction employs pyridines as substrates and nucleophilic sulfinates as sulfonylating agents. The reaction is driven by an electric current, which helps make reactive intermediates that sulfonylate the meta-position of the pyridine ring. A pyridyl radical is created in the proposed reaction mechanism. This radical then combines with the sulfonyl group, achieving the desired functionalization with high selectivity.
Benefits of Utilising Nucleophilic Sulfinates:
The strong reactivity and capacity to create stable sulfonylated products make nucleophilic sulfinates well-suited for this reaction. In contrast to other sulfonylation agents, sulfinates offer greater manageability and instant production, thereby reducing the need for hazardous chemicals. Their nucleophilic properties make it easy for them to couple with electrophilic intermediates that form during the electrochemical process. This leads to higher yields and better selectivity compared to traditional methods.
The procedure for conducting electrochemical Meta-C-H Sulfonylation experiments is detailed below:
The electrochemical Meta-C-H Sulfonylation process necessitates the use of the following materials and equipment:
1). Compounds derived from pyridine
2). Sulfinates have nucleophilic properties.
3). An electrochemical cell equipped with suitable electrodes.
4). Acetonitrile or DMF are examples of solvents.
5). An example of a supporting electrolyte is lithium perchlorate.
Usually, continuous current conducts the reaction. Initially, the solvent dissolves the pyridine and sulfinate compounds along with the supporting electrolyte. Subsequently, the mixture undergoes electrolysis, during which the electric current triggers the creation of the pyridyl radical. When the radical combines with the sulfinate, the sulfonylation process takes place, resulting in the formation of the required Meta-C-H Sulfonylation product. Synthetic applications.
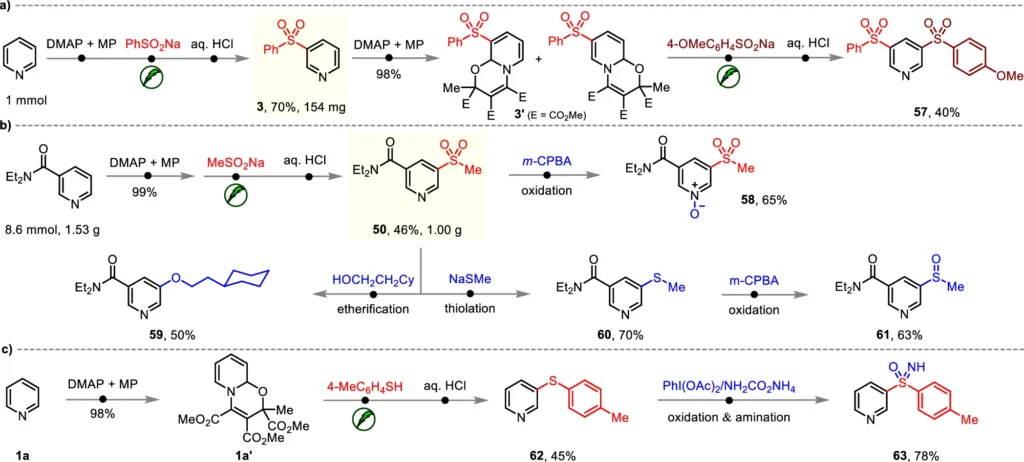
Enhancement of Reaction Conditions:
Maximizing yield and selectivity. The choice of solvent can have a significant impact on the reaction, with polar aprotic solvents frequently yielding optimal results. Electrochemical factors, such as the current density and the electrode material, also influence the effectiveness of the reaction. Optimizing these factors might result in significant enhancements in reaction results, necessitating the need to conduct experiments with various configurations.
Insights into the mechanisms:
Electrochemical Meta-C-H Sulfonylation is believed to involve multiple essential stages. Initially, the application of an electric current causes the pyridine to undergo oxidation, leading to the formation of a pyridyl radical. The radical then reacts with the nucleophilic sulfate, forming a sulfonylated intermediate. Further electron transfer processes achieve the outcome by stabilizing the sulfonyl group at the meta-position of the pyridine ring. The electrochemical cell is critical in this process because it provides the required energy to facilitate these conversions.
Obstacles and Constraints:
Although electrochemical Meta-C-H Sulfonylation has its benefits, it is not without difficulties. The experimental arrangement can influence electrochemical reactions, potentially impacting results with factors like electrode passivation or solvent degradation. Furthermore, the metaposition’s inherent complexity in terms of functionalization results in varying yields depending on the substrate used. Expanding the reaction for commercial use also poses challenges, as ensuring stable electrochemical conditions on a wider scale can be intricate.
Uses of Electrochemical Meta-C-H Sulfonylation:
This approach sulfonylates pyridines with substantial potential across diverse domains. Pharmaceuticals can use these chemicals as fundamental components to develop medications with enhanced pharmacokinetic properties. Sulfonyl groups can improve the stability and performance of polymers or electronic devices in the field of material science. This reaction is highly versatile and serves as a significant tool for scientists seeking to incorporate sulfonyl functionality into compounds that include pyridine. Mechanistic insights and plausible pathways.
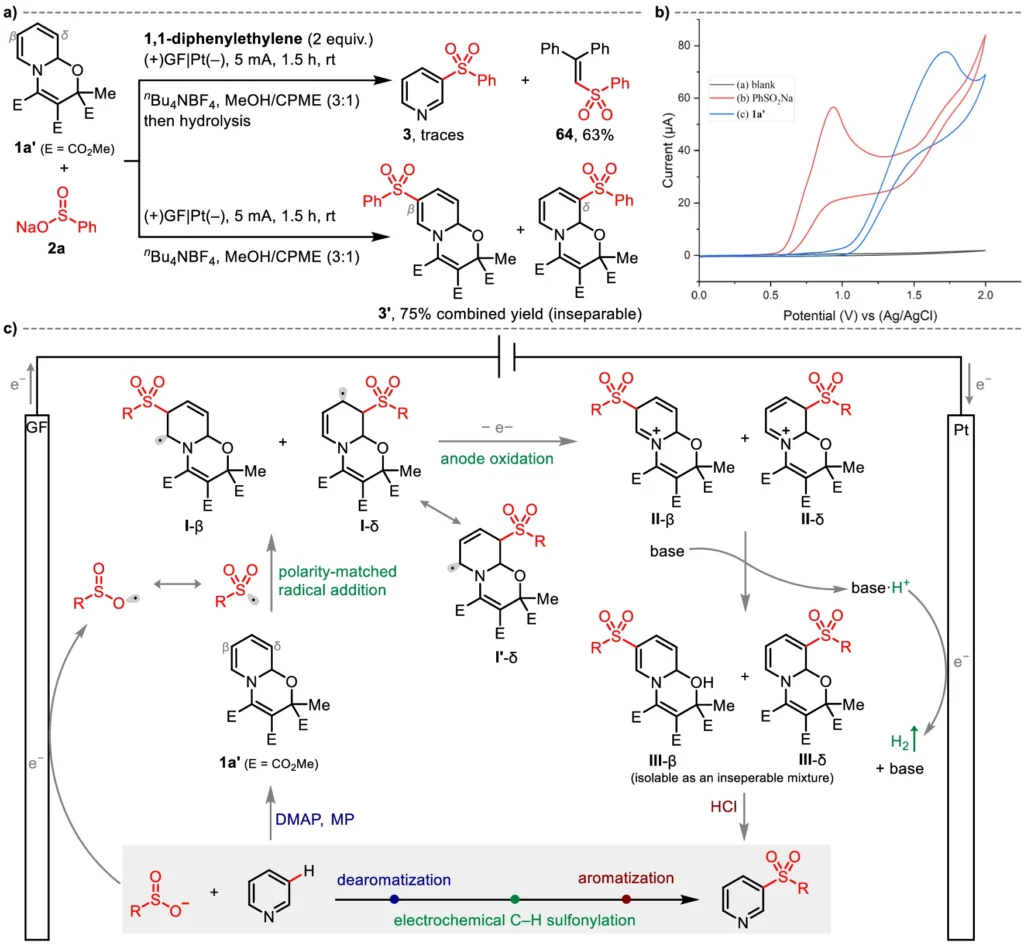
Comparative analysis comparing traditional methods:
Compared to traditional sulfonylation methods, the electrochemical method has several benefits, including safer reaction conditions, fewer unwanted byproducts, and maybe even lower costs. Electrochemistry frequently obviates the use of harsh reagents, hence diminishing the environmental impact. Nevertheless, the intricacy of establishing and enhancing electrochemical reactions might be a disadvantage, especially for individuals who are not acquainted with the method. However, the overall efficiency and scalability of this approach make it an intriguing alternative to traditional methods.
Prospects for Advancements in Electrochemical Organic Synthesis:
The electrochemical organic synthesis domain is undergoing tremendous advancements, with a continuous influx of novel techniques and technology. Further investigation could focus on broadening the range of substances that can undergo electrochemical meta-C-H sulfonylation or improving the procedure’s scalability for industrial purposes. Advancements in electrochemical apparatus, such as flow cells or novel electrode materials, have the potential to significantly improve the effectiveness and availability of these processes, facilitating their wider use in synthetic chemistry.
In conclusion:
An advanced way to change these important heterocycles is through electrochemical meta-C-H sulfonylation of pyridines with nucleophilic sulfinates. Scientists can achieve targeted sulfonylation in gentle environments using electrochemistry, creating new opportunities for the production of intricate compounds. We anticipate this methodology becoming a more valuable tool in both academic research and industrial applications as the field advances.
Frequently Asked Questions:
1). What does the term “Meta-C-H Sulfonylation” refer to?
Adding a sulfonyl group to the meta-position of an aromatic ring, like pyridine, is what meta-C-H sulfonylation is all about.
2). What role does electrochemistry play in enhancing sulfonylation reactions?
Electrochemistry uses an electric current to catalyze sulfonylation, resulting in a more favorable reaction and fewer unwanted byproducts than conventional techniques.
3). What are the benefits of using nucleophilic sulfinates?
Nucleophilic sulfinates have a strong reactivity, are more manageable, and produce stable sulfonylated products, making them well-suited for this particular reaction.
4). Is it possible to increase the size of this reaction for industrial applications?
While it is possible to scale up electrochemical reactions, achieving consistent conditions on a larger scale requires meticulous optimization, which can be quite challenging.
5). What are some potential uses for sulfonylated pyridines?
Pharmaceuticals, material science, and other domains that require improved stability and solubility utilize sulfonylated pyridines.
For more chemistry blogs, visit chemistry Master