Table of Contents
Researchers in the field of electrocatalysis have been investigating molybdenum sulfide (MoS2) as a potential efficient electrocatalyst for hydrogen evolution. Molybdenum sulfide, a type of compound known as a transition metal dichalcogenide, displays exceptional catalytic characteristics due to its distinct allotropes and topologies. Comprehending the connections between activity and stability in various Molybdenum sulfide allotropes is essential for maximizing their efficiency in hydrogen evolution processes (HER).
An overview of molybdenum sulfide electrocatalysts:
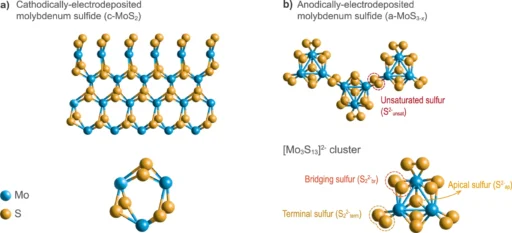
Electrocatalysts are substances that augment the speed of electrochemical reactions without undergoing consumption. Electrocatalysts are crucial components in renewable energy technologies, particularly in processes such as HER, which is essential for the production of environmentally friendly hydrogen fuel. The several forms of molybdenum sulfide have shown great potential as a suitable option for the hydrogen evolution reaction (HER) because of their adjustable characteristics and cost efficiency. Chemical structure of studied MoSx allotropes.
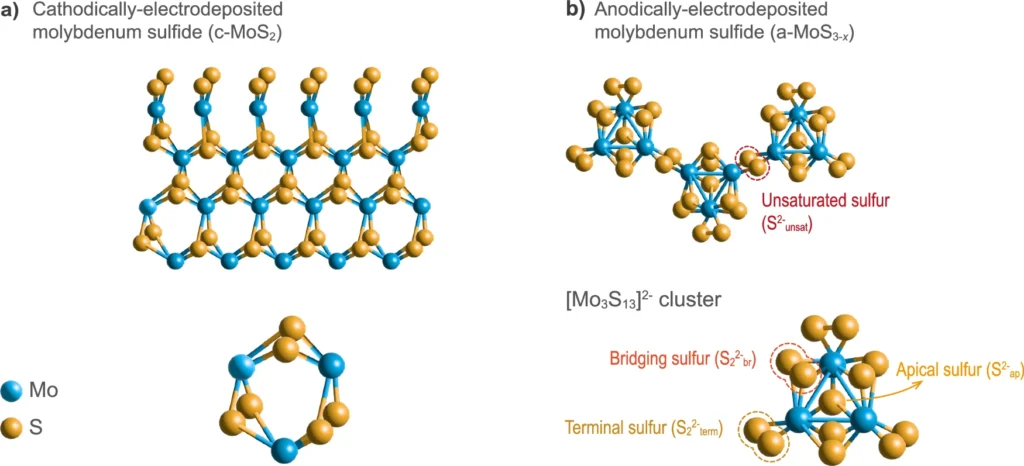
Allotropes of Molybdenum Sulfide:
Molybdenum sulfide (MoS2) can exist in many allotropes, each characterized by unique atomic configurations. The two most prevalent allotropes that are significant in electrocatalysis are the 2H and 1T phases. The 2H phase is composed of stacked layers that are bound together by weak van der Waals interactions. In contrast, the 1T phase has a deformed lattice structure and exhibits metallic conductivity. The electrochemical performance of MoS2 is greatly influenced by these structural variations.
Correlation between Activity and Stability:
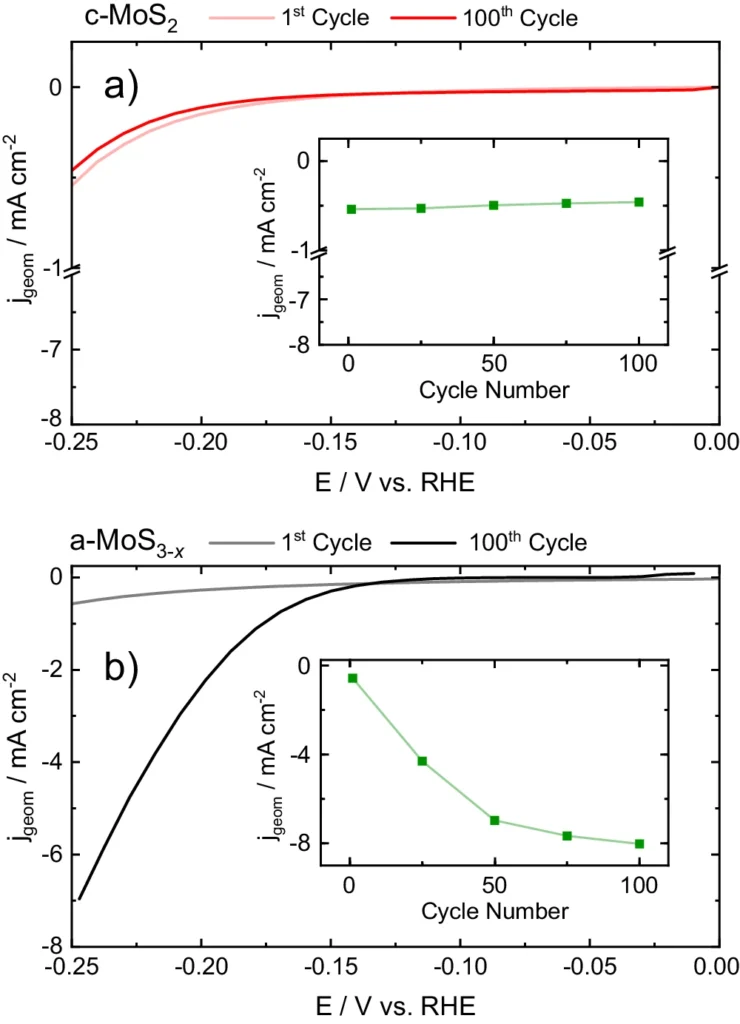
The activity-stability connection in electrocatalysis pertains to the intricate equilibrium between catalytic activity and structural stability. The connection for MoS2 electrocatalysts is affected by parameters such as crystallinity, surface imperfections, and exposure to reaction conditions. Comprehending the behavior of various allotropes under varied conditions is crucial for the development of long-lasting electrocatalysts. Electrochemical activity of MoSx allotropes.
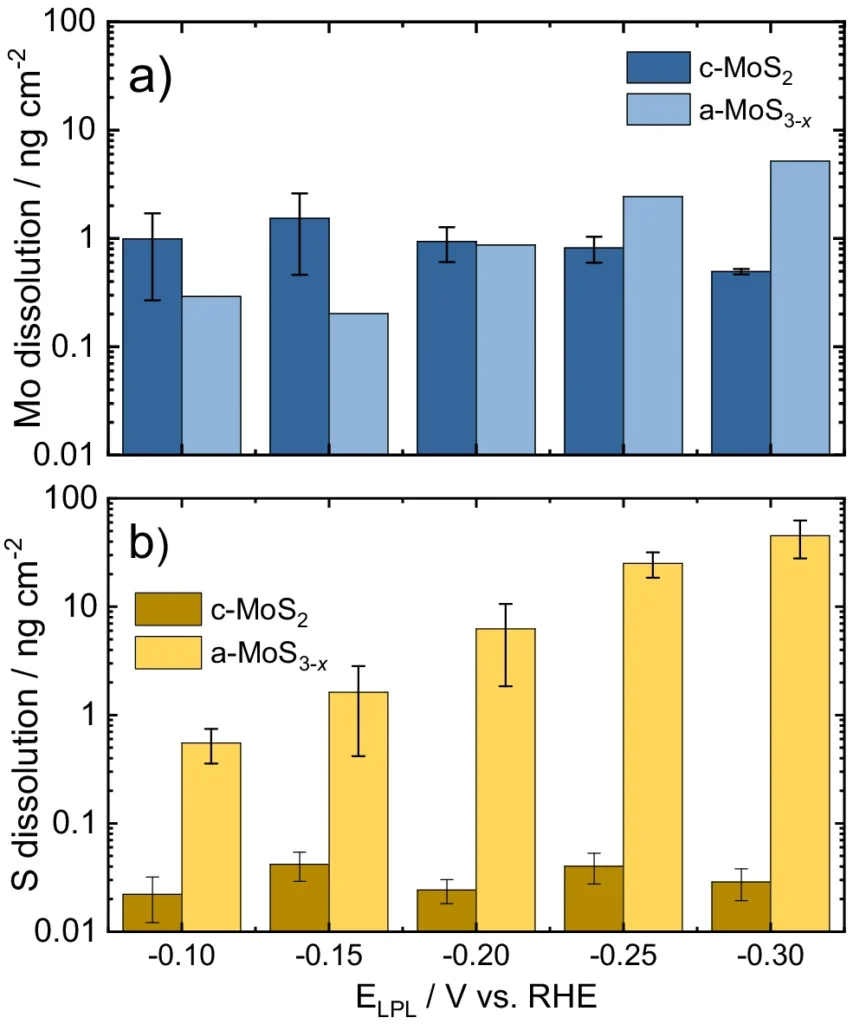
Graphical representation of total integrated dissolution of (a) Mo (blue) and (b) S (yellow) as a function of the LPL. For electrochemical protocol,
Methods for Characterization:
Characterization techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) are essential for investigating the structural and chemical features of MoS2. These methods clarify the influence of allotropes on catalytic activity and stability, offering valuable insights for the design and optimization of catalysts.
Hydrogen Evolution Reaction (HER):
The Hydrogen Evolution Reaction (HER) is a process in which protons are reduced to generate hydrogen gas. MoS2 functions as a catalyst by offering active sites for the adsorption of protons and the subsequent production of hydrogen. The selection of MoS2 allotrope has a considerable impact on the kinetics and efficiency of HER. The 1T phase is generally preferred because it has better performance because of its improved charge transfer capabilities.
Empirical Results:
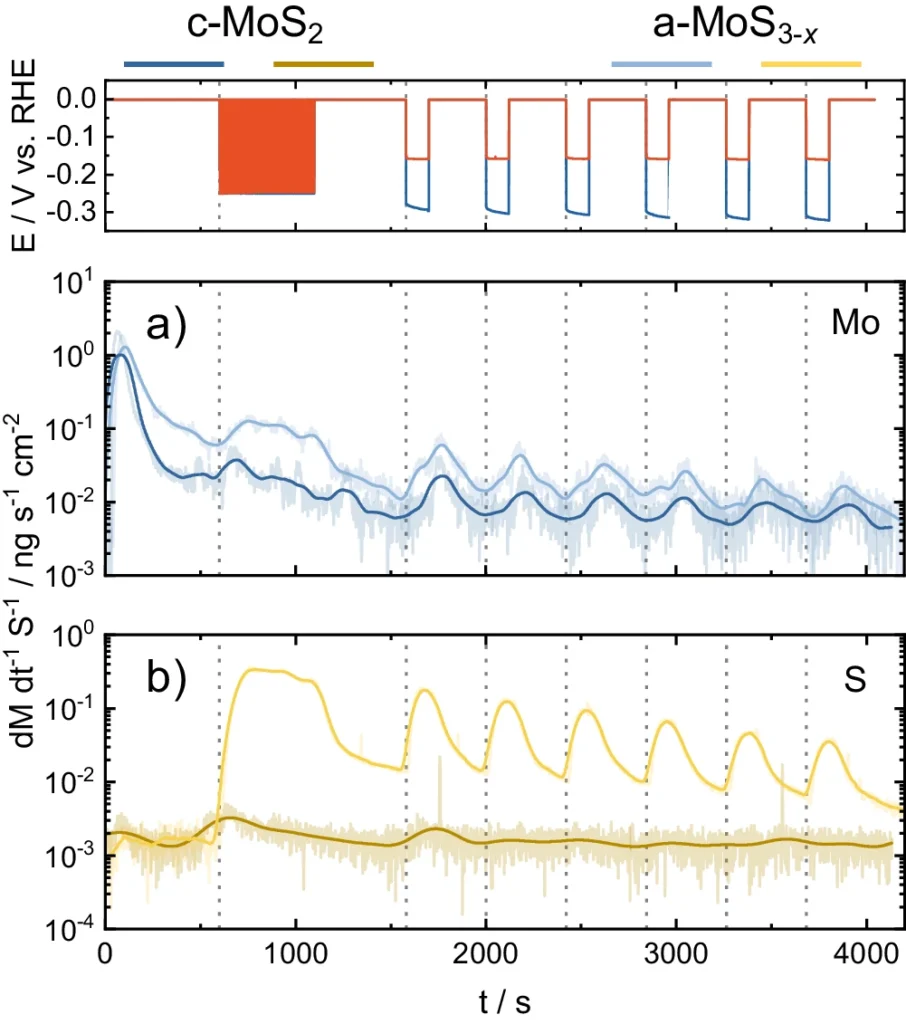
Recent investigations have shown that the 1T phase of MoS2 generally exhibits more hydrogen evolution reaction (HER) activity in comparison to the 2H phase. Nevertheless, the 2H phase might provide superior long-term stability in specific circumstances. Scientists are now investigating various synthetic techniques and surface modifications to customize MoS2 catalysts to achieve the best balance between activity and stability. Online ICP-MS data during HER start-up/shutdown.
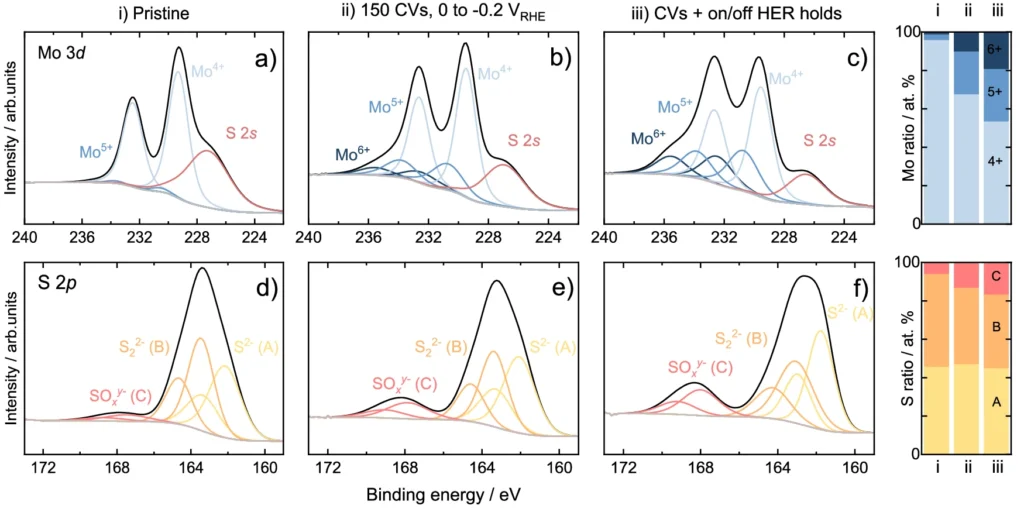
Ex-situ XPS data on Mo3S13-NCNT before/after HER experiments.
Variables Influencing Stability:
The stability of MoS2 catalysts can be affected by environmental conditions such as pH, temperature, and the makeup of the electrolyte. In addition, surface changes such as doping with foreign atoms or nanostructuring can improve stability by reducing degradation mechanisms such as sulfur loss or lattice reformation.
Enhancing the efficiency of Molybdenum sulfide catalysts:
Researchers are exploring methods such as defect engineering, heteroatom doping, and nanostructuring to optimize the performance of Molybdenum sulfide electrocatalysts by increasing their catalytic activity without compromising stability. It is essential to create catalysts that achieve a balance between their activity and stability to effectively use them in renewable energy technologies.
Practical Uses in the Real World:
Electrocatalysts based on MoS2 show potential for other applications beyond hydrogen evolution reaction (HER), such as water splitting, electrochemical sensors, and energy storage devices. Nevertheless, there are still obstacles to overcome when it comes to expanding output, enhancing longevity, and incorporating MoS2 catalysts into operational devices for extensive utilization in sustainable energy systems.
In conclusion:
Ultimately, the activity-stability relationships of molybdenum sulfide electrocatalysts, which depend on the allotrope, play a crucial role in the progress of electrocatalysis. Researchers are studying the complex relationship between MoS2 structures and electrochemical performance to create strong catalysts that will help transition to a hydrogen-based economy.
Frequently Asked Questions:
1. How does MoS2 compare to other electrocatalysts in terms of hydrogen evolution?
MoS2 has comparable performance and cost benefits in comparison to noble metal catalysts like platinum, rendering it a possible substitute for HER.
2. What difficulties arise while attempting to synthesize and increase the size of MoS2 catalysts?
Obtaining a consistent shape and ensuring the presence of only one phase are important difficulties in producing MoS2 electrocatalysts in large quantities for industrial use.
3. Is it possible to customize MoS2 for specific catalytic uses other than hydrogen evolution?
Indeed, the characteristics of MoS2 can be adjusted by making changes to its structure and modifying its surface to accommodate a wide range of electrochemical reactions.
4. What strategies can be employed to resolve the stability concerns associated with MoS2 catalysts for their practical implementation?
Researchers are investigating surface engineering, protective coatings, and advanced catalyst design methodologies to improve the durability and lifespan of catalysts based on MoS2.
5. What are the ecological advantages of utilizing MoS2 electrocatalysts?
MoS2-based catalysts facilitate environmentally friendly hydrogen synthesis without dependence on limited or costly resources, hence supporting sustainable energy solutions.
For more chemistry blogs, visit chemistry Master