Table of Contents
Overview:
Nature’s captivating substance, ice, is more than just solidified water. Hydrogen bonding, a weak link that significantly influences the structure and behavior of water molecules, controls the intricate properties of this material. But what happens when we confine ice to incredibly small regions, just a few nanometers in size? Within these tiny spaces, ice exhibits peculiar and captivating behavior, leading to the emergence of phenomena like quasi-one-dimensional hydrogen bonding. This article examines the phenomenon by investigating the distinct properties of ice confined at the nanoscale and the consequences of quasi-one-dimensional hydrogen bonding.
Analyzing the Concept of Hydrogen Bonding in Ice:
To understand the unique characteristics of nanoconfined ice, it is critical to first acquire a fundamental understanding of hydrogen bonding in ice on a larger scale. Hydrogen bonding maintains the arrangement of water molecules in a crystalline structure, creating ice. The formation of these bonds occurs when the positive-charged hydrogen atom from one water molecule attracts the negatively-charged oxygen atom from another water molecule. A tetrahedral arrangement is made when chemical bonds are formed. This is what gives ice its unique properties, such as its lower density compared to liquid water.
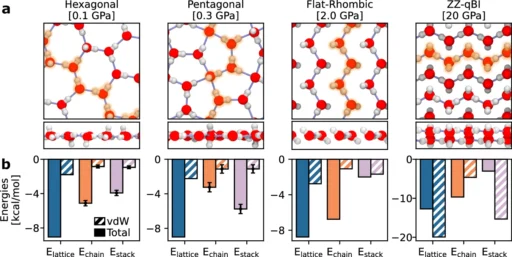
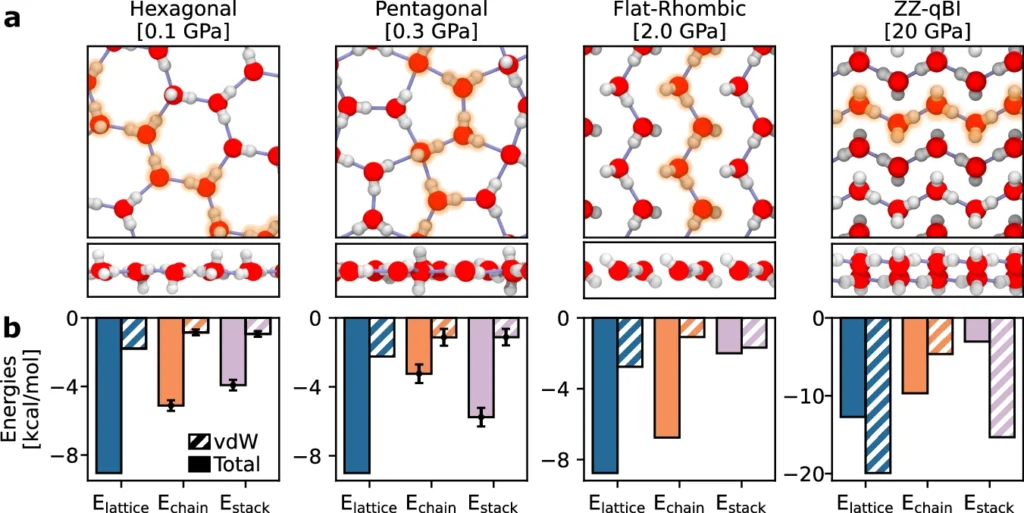
Hydrogen bonds have significantly lower strength in comparison to covalent bonds, although they possess sufficient strength to generate the inflexible arrangement of ice. These connections within bulk ice form a three-dimensional network that determines the ice’s stability and characteristics. Nanoconfined ice phases and the role of vdW stacking of hydrogen-bonded chains.
Nanoconfinement: Exploring uncharted territory
Nanoconfinement is the act of limiting materials to tiny places, usually at the nanoscale scale. The molecular structure of ice can undergo significant changes when it forms within restricted areas, such as carbon nanotubes, nanopores, or between layers of clay. Nanoconfinement modifies molecular interactions, resulting in novel characteristics that are absent in bulk materials.
Nanoconfinement in water and ice can cause significant changes in hydrogen bonding, phase behavior, and ice’s physical structure. For instance, trapped ice in nanoscale pores may not adopt the typical hexagonal structure of bulk ice. Instead, it may take on other crystalline or amorphous forms.
Quasi-One-Dimensional Structures:
In a quasi-one-dimensional structure, molecules or atoms primarily move along one dimension, exhibiting minimal flexibility in the other two dimensions. Several physical systems, like specific categories of polymers or the transmission of electrons in tiny wires, exhibit this particular configuration.
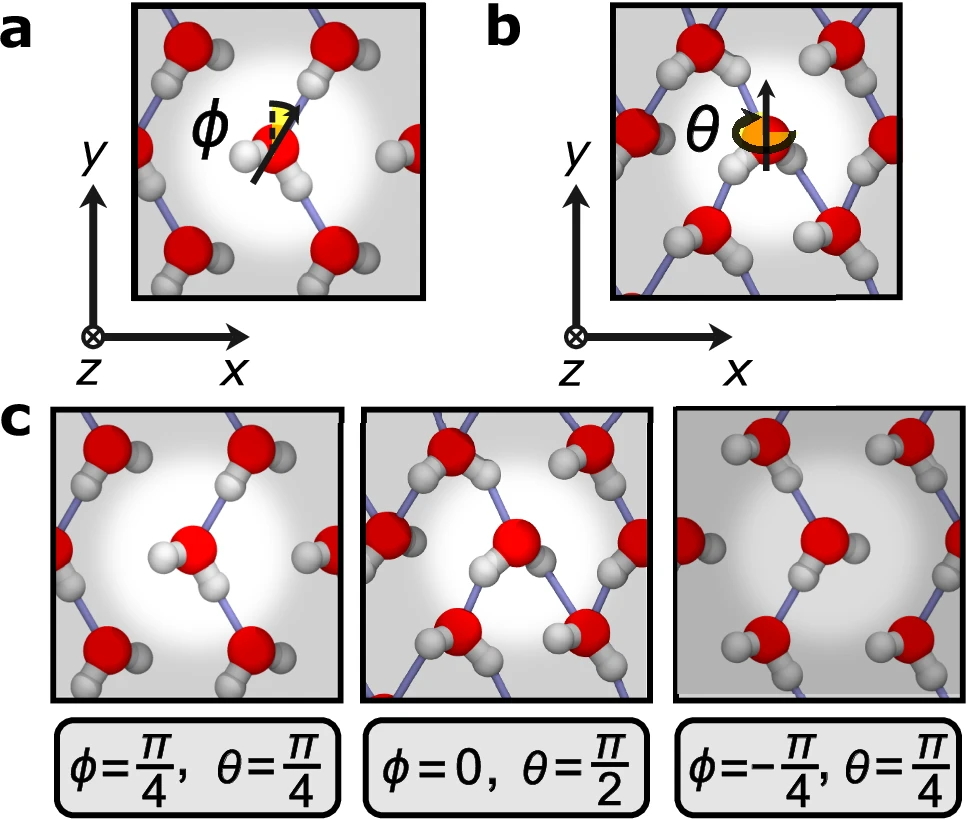
In nanoconfined ice, water molecules align in a linear or chain-like structure, leading to the formation of hydrogen bonds primarily along that line. The linear arrangement of molecules can result in distinct physical characteristics, such as alterations in the intensity, alignment, and movement of the hydrogen bonds. Depiction of the orientations of water molecules.
Creation of Ice in Nanoconfinement:
When water is constrained within nanoscale cavities, its transformation into ice deviates from the typical process observed in larger volumes of water. The restricted environment can influence the alignment and bonding of water molecules, resulting in ice structures that differ from those created in broader regions.
Water molecules organize themselves in such restricted spaces to optimize hydrogen bonding, often resulting in quasi-one-dimensional structures. Water enclosed within a carbon nanotube can arrange itself into a linear chain of water molecules, with each molecule connected to its neighboring molecules through hydrogen bonds.
Investigation of Hydrogen Bonding in Nanoconfined Ice:
The nanoscale confinement of molecules significantly alters the characteristics of hydrogen bonding in ice. The quasi-one-dimensional structure results in a hydrogen-bonding network that is more focused and frequently more powerful than bulk ice. The system’s decreased dimensionality reduces the probability of hydrogen bonds forming the intricate networks observed in bulk ice. Instead, the bonds become more linear and predictable.
Molecular dynamics simulations and experimental experiments demonstrate that quasi-one-dimensional hydrogen bonding can significantly alter the physical properties of ice. For instance, compared to ice in its normal state, the restricted ice may exhibit distinct characteristics like thermal conductivity, electrical properties, or mechanical strength. Hydrogen bonded switching behavior.
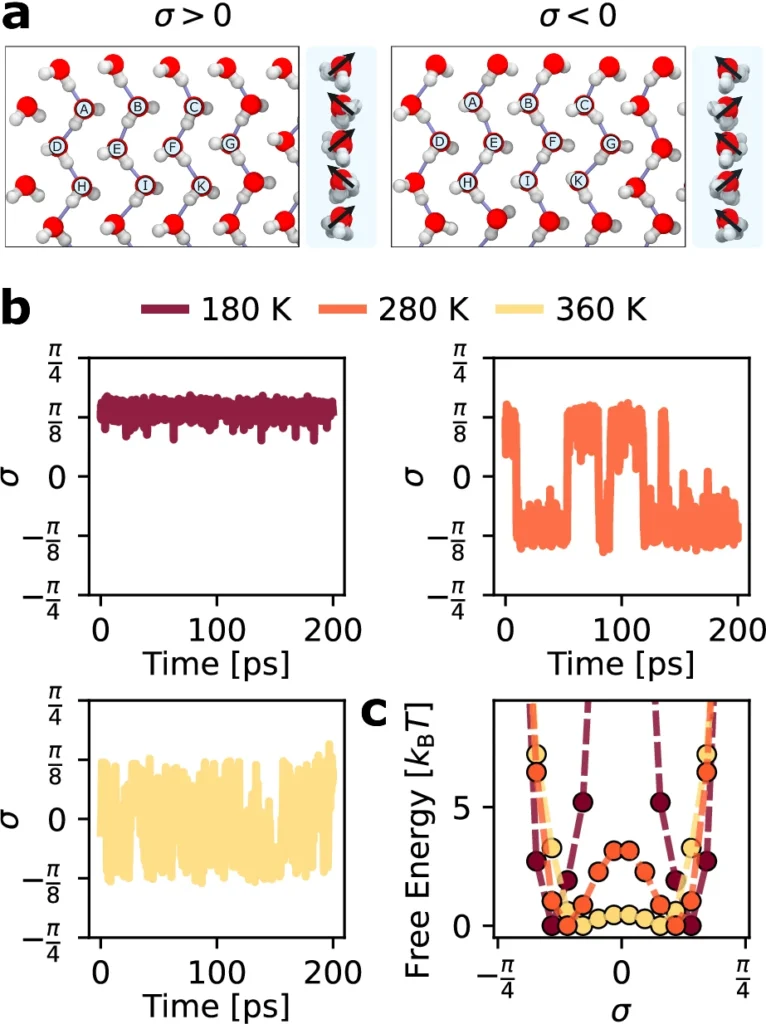
Ice Confined at the Nanoscale: Thermodynamic Characteristics
The restricted space similarly influences the thermodynamic characteristics of ice limited at the nanoscale, including its points of melting and freezing. Compared to ice in bulk form, the phase transitions of ice confined at the nanoscale frequently alter. In particular, the melting point is usually lower due to the heightened surface energy and the limitations on molecule movement.
Furthermore, the changes in entropy and enthalpy during phase transitions in nanoconfined ice may differ significantly from bulk ice. This is because the quasi-one-dimensional hydrogen bonding network leads to the formation of a highly organized structure, resulting in a decrease in the system’s entropy.
The characteristics of ice confinement at the nanoscale are described:
Nanoconfined ice’s structural characteristics can significantly differ from those of its bulk form. While ice in large quantities usually takes the shape of a hexagonal lattice (known as ice Ih), frozen that is confined at the nanoscale can have several structures, either crystalline or amorphous, depending on the type of confinement. For instance, ice can exhibit high anisotropy when trapped in small pores or sandwiched between layers of other substances, indicating that its characteristics vary depending on the direction of measurement.
Under certain circumstances, confinement can result in the creation of wholly novel ice phases that are not present in larger quantities. These phases may demonstrate distinct qualities, such as increased mechanical resilience or modified optical attributes.
Implications of Quasi-One-Dimensional Hydrogen Bonding:
The presence of quasi-one-dimensional hydrogen bonding in nanoconfined ice has significant consequences for the stability and behavior of ice in confined spaces. The orientation and intensity of these connections can render nanoconfined ice more stable under specific circumstances compared to bulk ice, hence potentially inhibiting its melting even at temperatures that would typically cause bulk ice to turn into a liquid state.
Practical scenarios, such as the safekeeping and transportation of extremely cold items or the conservation of biological specimens, could utilize the improved stability of this phenomenon. Moreover, the modified hydrogen bonding network could impact the interaction between nanoconfined ice and other substances, potentially resulting in novel findings in the field of materials science. Temperature dependence of hydrogen bonding
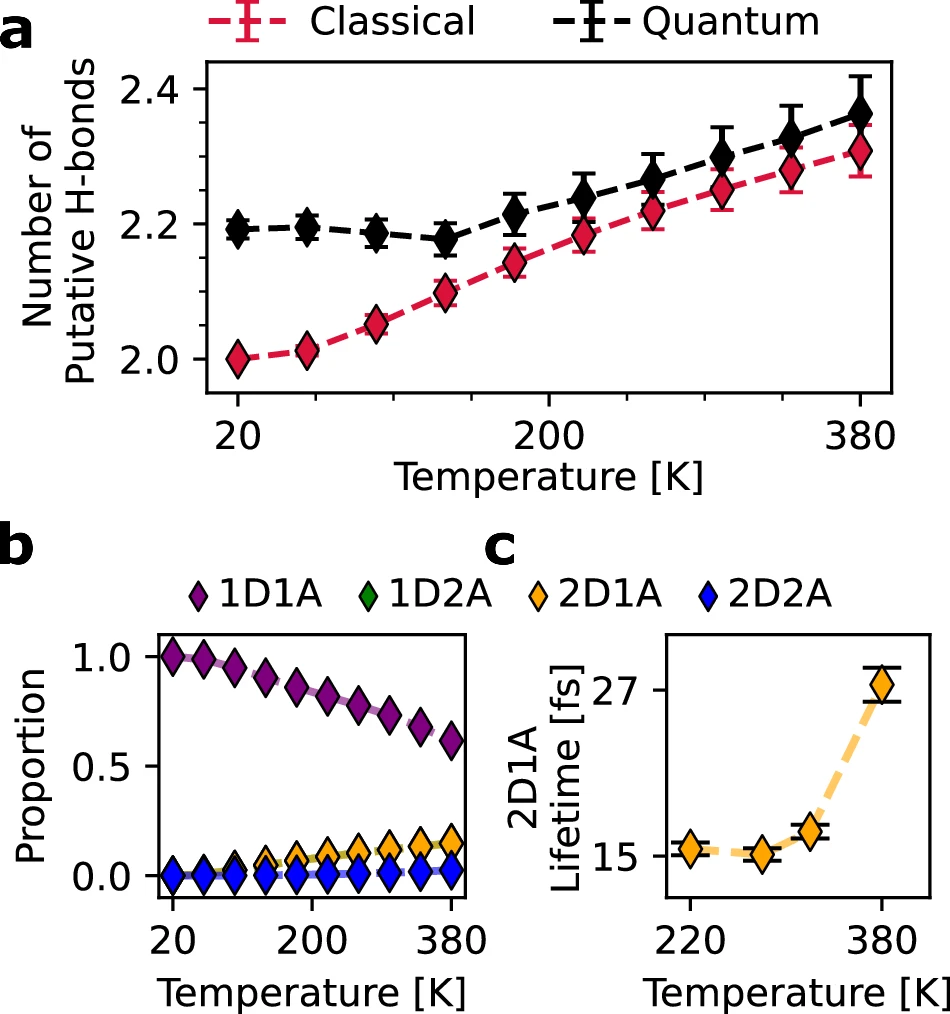
photo credit .a The average number of putative/geometric hydrogen bonds across the range of temperatures in which the flat-rhombic phase is stable using classical and quantum simulations. The error bars show the standard error of the mean, as computed by block averaging over 10 blocks. b The proportion of water molecules of each hydrogen bonding motif (defined in the text). The 1D2A proportions are perfectly aligned with the 2D1A proportions.
c The 2D1A motif’s lifetime remains extremely short across the range of temperatures in which it is observed. The error bars show the standard error of the mean, computed across all instances of hydrogen bonding. Dashed lines serve as a visual guide for the eye. Source data are provided as a Source Data file.
Utilizations of Nanoconfined Ice:
Nanoconfined ice shows promise in several domains, including cryopreservation and nanotechnology. Cryopreservation can harness the distinct characteristics of nanoconfined ice to enhance the preservation of biological tissues at low temperatures. This method reduces the likelihood of ice crystal formation, which can be harmful to cells.
In the field of nanotechnology, manipulating ice’s structure and properties at the nanoscale has the potential to facilitate the creation of novel materials with customized attributes. For instance, we could use nanoconfined ice to develop innovative filtration systems or as a medium for precise chemical reactions.
Furthermore, the examination of nanoconfined ice is pertinent to natural phenomena, such as the characteristics of water within the cavities and cracks of rocks or the formation of ice in biological structures such as cell membranes.
Obstacles and Constraints:
Although nanoconfined ice has enormous potential, it is not without its challenges. It can be difficult to generate and detect ice at extremely small sizes, and the methods used to investigate it—such as high-resolution microscopy or spectroscopy—frequently require advanced equipment and specific conditions.
Conceptually, simulating the actions of ice confined at the nanoscale has its own set of difficulties. Advanced computational approaches and extensive computer power are necessary to study the intricate relationship between hydrogen bonding, confinement, and molecular dynamics.
Prospects for Future Research:
The investigation of ice contained at the nanoscale is a rapidly progressing area of research, characterized by frequent advancements in methodology and discoveries. We expect subsequent studies to prioritize the development of more effective techniques for viewing and controlling nanoscale ice. We will also focus on discovering new types of ice that only appear under these restricted conditions.
Furthermore, as our understanding of quasi-one-dimensional hydrogen bonding advances, it has the potential to provide novel perspectives on the behavior of other substances and systems that rely on hydrogen bonding. This research has the potential to have significant effects on various domains, including chemistry, physics, biology, and materials science.
Case Studies: Concrete Instances of Nanoconfined Ice
At the nanoscale, various distinct instances exemplify the distinctive characteristics of ice. Ice trapped inside carbon nanotubes exhibits a quasi-one-dimensional arrangement that significantly alters its thermal and mechanical properties.
Likewise, when ice is trapped between layered minerals or clays, it can create distinct crystalline formations that are not typically observed in large quantities of ice. These restricted ice phases can influence the mechanical properties of the primary material, potentially finding application in the field of materials research.
Within cell membranes or other cellular structures in biological systems, nanoconfined ice plays a role in processes like freeze tolerance in particular organisms. Temperature dependence of the dielectric response.
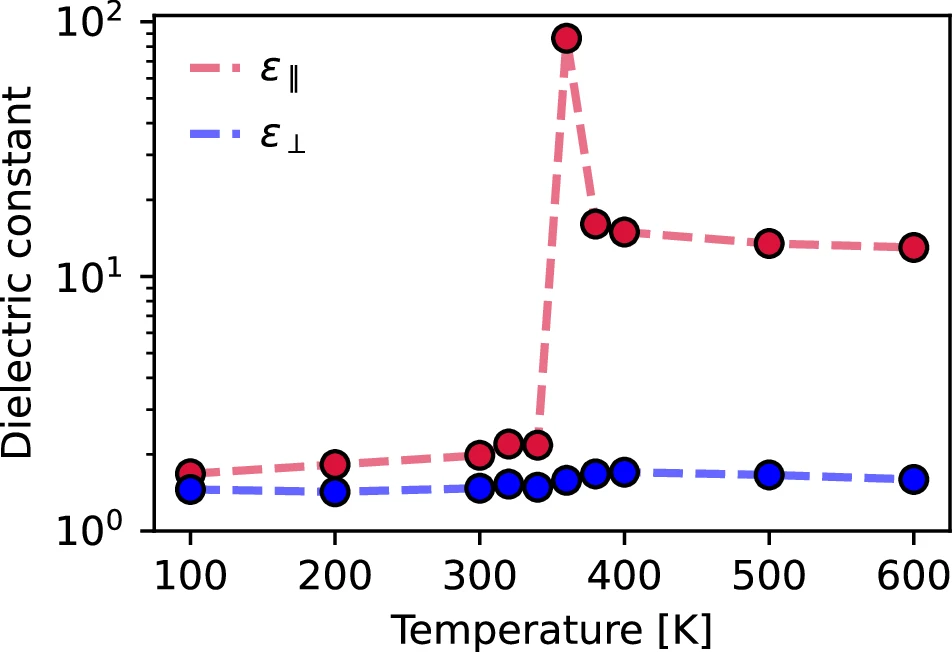
The broader implications of quasi-one-dimensional hydrogen bonding are worth considering:
The exploration and analysis of quasi-one-dimensional hydrogen bonding in nanoconfined ice have wider consequences that extend beyond the mere examination of ice. This phenomenon offers a novel viewpoint on hydrogen bonding in a broader sense, potentially impacting our comprehension of other systems in which hydrogen bonding is crucial, such as proteins, DNA, and other biological molecules.
Furthermore, the knowledge gained from nanoconfined ice research has the potential to be used in several disciplines, potentially leading to the development of novel materials, technologies, and understandings in various scientific domains.
In conclusion:
The phenomenon of quasi-one-dimensional hydrogen bonding in nanoconfined ice is a captivating occurrence that defies our conventional comprehension of ice and hydrogen bonding. The distinct attributes of ice contained at the nanoscale, such as its modified thermodynamic and structural properties, have substantial ramifications across various scientific disciplines. As more study progresses in this field, we can expect to discover additional information regarding the enigmas of nanoconfined ice and the wider consequences of quasi-one-dimensional hydrogen bonding.
Frequently Asked Questions:
1). What is nanoconfined ice?
Nanoconfined ice is ice that forms in very small spaces, like carbon nanotubes or nanopores. The molecular structure and properties of this ice may be very different from regular ice which is found in larger amounts.
2). What effect does nanoconfinement have on hydrogen bonding?
Nanoconfinement can create hydrogen bonding networks that are almost one-dimensional, with hydrogen bonds that are more linear and directed than in bulk ice. This leads to the emergence of distinct physical and chemical properties.
3). What potential applications can ice with nanoscale restrictions find?
The unusual features and stability of nanoconfined ice make it a promising candidate for various applications, including cryopreservation, nanotechnology, and materials research.
4). What experimental methods are used to study nanoconfined ice?
Researchers employ advanced techniques like high-resolution microscopy, spectroscopy, and molecular dynamics simulations to study nanoconfined ice. These methods enable researchers to examine and understand the structure and behavior of ice at the nanoscale.
5). What are the potential future outcomes of studying in this field?
The focus of future research will be on creating new ways to look at ice that is confined at the nanoscale level, finding structures of ice that were not known before, and applying what was learned in these studies to other fields, like materials science and biology.
For more chemistry blogs, visit chemistry Master