Table of Contents
Overview of Nuclear Spin Polarization:
The technique of nuclear spin polarization is an advanced method that has significant ramifications in various sectors, such as medical diagnostics, chemical analysis, and industrial operations. The nuclear Spin Polarization technique improves the sensitivity of magnetic resonance imaging (MRI) and nuclear magnetic resonance (NMR) spectroscopy by aligning the nuclear spins within a substance. An intriguing application is the enhancement of nuclear spin polarization in lactic acid by the interchange of parahydrogen-polarized protons. This article offers a comprehensive examination of the procedures, mechanisms, and possible uses of this captivating approach.
An Exploration into Nuclear Spin Polarization:
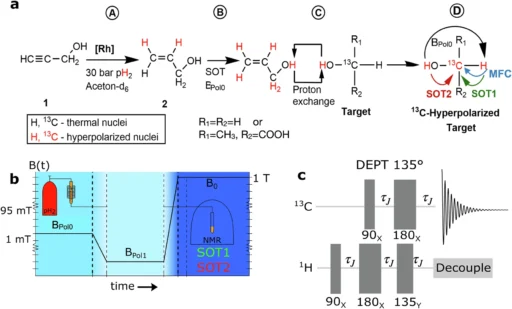
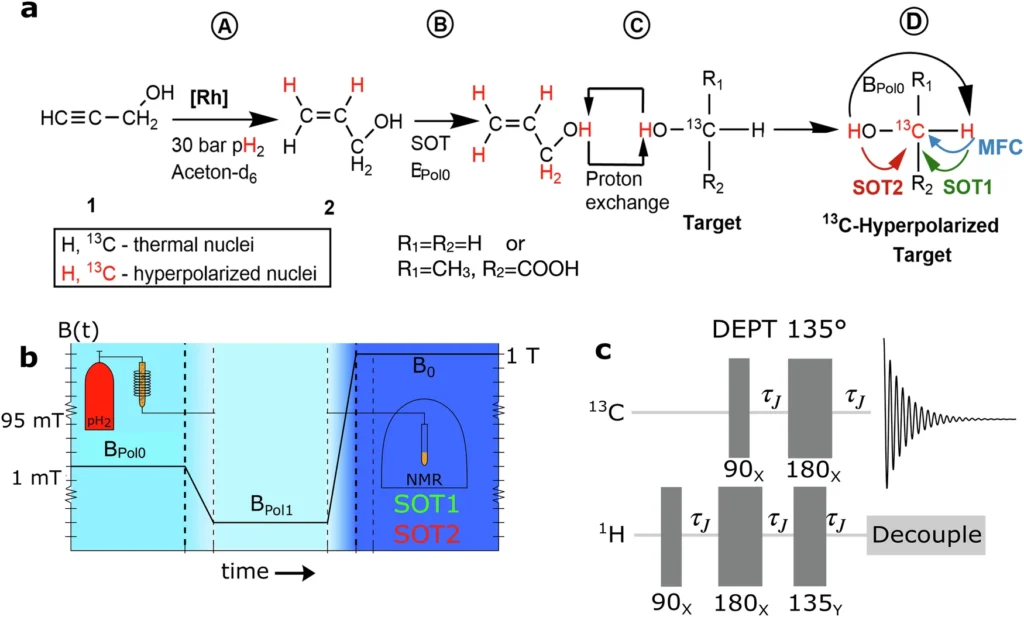
Nuclear spin polarization refers to the process of aligning the nuclear spins of atoms in a material, which leads to an increase in its magnetic characteristics. This alignment greatly enhances the signal-to-noise ratio in magnetic resonance techniques, enabling the acquisition of more accurate and sensitive measurements. The historical advancement of nuclear spin polarization techniques has had a significant impact on areas such as medical imaging and chemical analysis. Dynamic nuclear polarization (DNP) and parahydrogen-induced polarization (PHIP) are advanced techniques that have become valuable tools in this field, providing distinct advantages and applications. Schematic view of PHIP-X, magnetic field cycling (MFC,) and RF pulse sequences used in simulations and experiments.
Characteristics and Significance of Lactic Acid:
Lactic acid, with the chemical formula C3H6O3, is an organic acid that occurs naturally and has diverse applications in the industrial and medicinal sectors. Chemically, the presence of a hydroxyl group and a carboxyl group distinguishes it, enhancing its reactivity and adaptability. From a biological perspective, lactic acid is essential for metabolic processes, namely anaerobic respiration. Muscle cells create lactic acid during severe exercise. This substance has a wide range of industrial applications, including food preservation, cosmetics, and biodegradable plastic production. From a medical perspective, lactic acid plays a crucial role in the examination of metabolic disorders and cancer, which makes it a perfect option for polarization investigations.
An Overview of Parahydrogen:
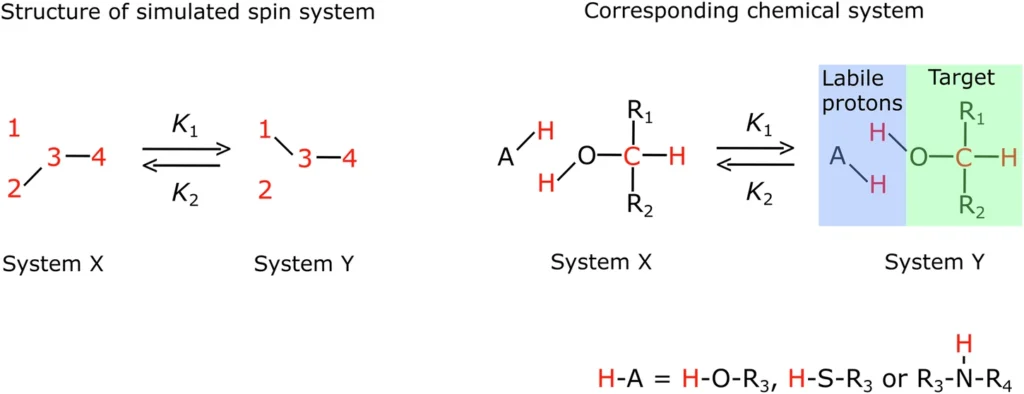
Parahydrogen, one of the two spin isomers of hydrogen, has distinctive magnetic characteristics due to its aligned nuclear spin polarization. Orthohydrogen and parahydrogen differ in their hydrogen nuclei’s spin orientation. While orthohydrogen has parallel spins, parahydrogen has antiparallel spins, leading to a state of lower energy. The parahydrogen feature makes it very suitable for polarization transfer in PHIP. Producing and purifying parahydrogen entails reducing hydrogen gas to extremely low temperatures and utilizing catalysts to convert orthohydrogen into parahydrogen, resulting in a high level of purity that is critical for successful polarization. Scheme of the spin system simulated.
The Parahydrogen-Induced Polarization (PHIP) Mechanism:
PHIP uses parahydrogen’s distinctive spin characteristics to amplify the nuclear polarization of different molecules. Chemically introducing parahydrogen to a substrate, such as through a hydrogenation reaction, significantly enhances the NMR signals of the resulting product. This boost arises from the transfer of spin order from parahydrogen to the nuclei of the substrate, resulting in the alignment of their spins. Compared to other polarization techniques, PHIP has various advantages, such as its simplicity, cost-effectiveness, and ability to obtain high levels of polarization without requiring severe conditions like cryogenic temperatures or high magnetic fields.
Polarization Exchange Process:
Catalysts mediate a sequence of chemical processes that exchange parahydrogen-polarized protons with lactic acid. Under specific conditions, the parahydrogen molecules transfer their spin polarization to the protons of lactic acid via interaction. Several factors, including the catalyst selection, temperature, pressure, and the purity level of parahydrogen, determine the outcome of this exchange. Optimization strategies aim to maximize the efficiency of this transfer, guaranteeing that a substantial fraction of lactic acid molecules get polarized. 13C-methanol hyperpolarized with PHIP-X.
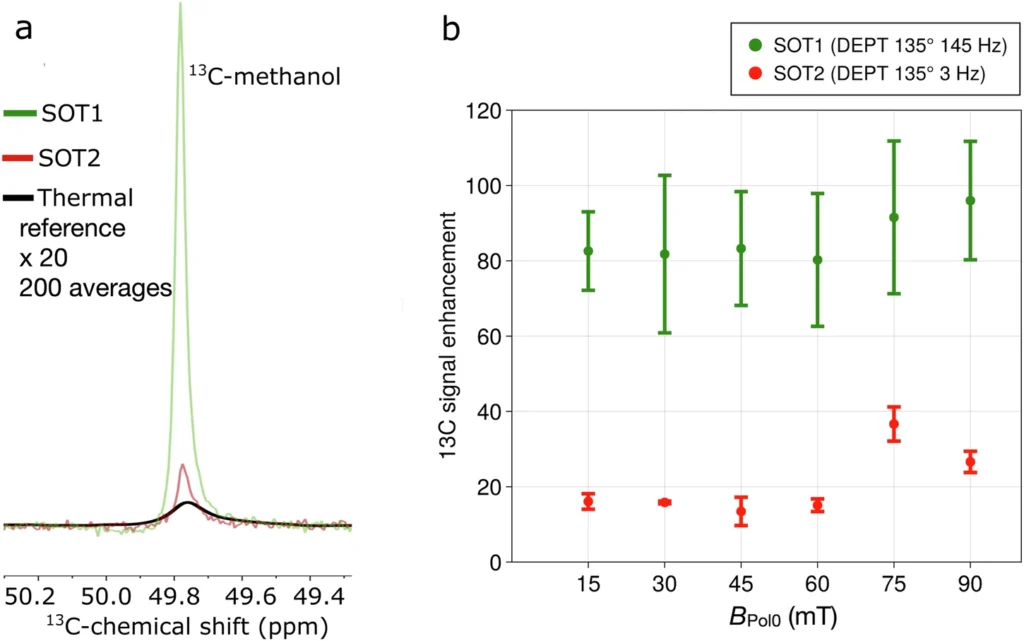
PHIP-X hyperpolarized 13C3-lactic acid (LA).
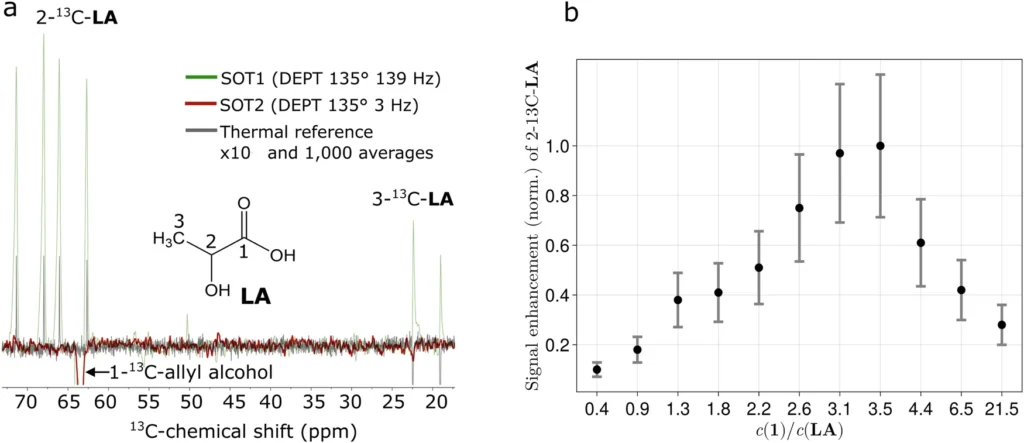
Polarization Experimental Configuration:
To achieve efficient lactic acid polarization by PHIP, a carefully planned experimental arrangement is required. The key elements comprise a parahydrogen source, a hydrogenation reactor, and an appropriate catalyst. A catalyst inside the reactor subjects the lactic acid to parahydrogen, ensuring precise control over temperature and pressure. The process generally entails lowering the temperature of hydrogen gas to generate parahydrogen, followed by purification, and then introducing it to lactic acid under ideal conditions to promote polarization exchange.
Methods and procedures for measuring and analyzing data:
NMR spectroscopy primarily quantifies nuclear spin polarization in lactic acid. Hyperpolarized NMR techniques allow for the identification of greatly amplified signals, which yield precise information on the levels of polarization. The analysis of these results entails comparing the NMR signals of polarized lactic acid with those of non-polarized samples, to ensure the data’s precision and dependability. Executing this procedure requires advanced machinery and specialized knowledge to decipher the intricate spectra produced during the examination.
Difficulties in Polarizing Lactic Acid:
Although there are potential advantages, the process of polarizing lactic acid through parahydrogen-polarized protons poses many difficulties. The technical challenges include the need to ensure the polarized state’s durability and achieve effective polarization transmission. Currently, the existing technology faces limitations in terms of scaling up and replicating its results. To tackle these issues, it is necessary to continuously conduct research to enhance catalysts, optimize experimental settings, and devise more effective techniques for the production and utilization of parahydrogen.
Using Polarized Lactic Acid in Medicine:
Because of its increased capabilities, the use of polarized lactic acid in MRI and NMR imaging has significant implications for medical diagnostics. This approach can facilitate the early detection and monitoring of illnesses by providing sharper images and more comprehensive metabolic information. For example, lactic acid polarization can improve tumor detection in cancer diagnostics, leading to more precise evaluations of tumor metabolism and treatment effectiveness. Moreover, this method is highly beneficial for investigating metabolic problems as it enables more accurate quantification of lactic acid concentrations and metabolic pathways.
Applications exist in the industrial and chemical sectors:
Polarized lactic acid enables real-time monitoring of processes in the chemical industry, offering significant insights into reaction dynamics and mechanisms. This can lead to the development of more effective catalysts and the improvement of reaction conditions, thereby increasing the overall efficiency of chemical processes. Polarized lactic acid in the pharmaceutical sector improves drug metabolism and bioavailability assessment, thereby facilitating the creation of more efficient and focused therapies. The heightened sensitivity of NMR spectroscopy in industrial applications allows for improved precision and optimization of manufacturing processes. 1H and 13C polarization of the fixed proton and carbon in the target molecule during a PHIP-X experiment.
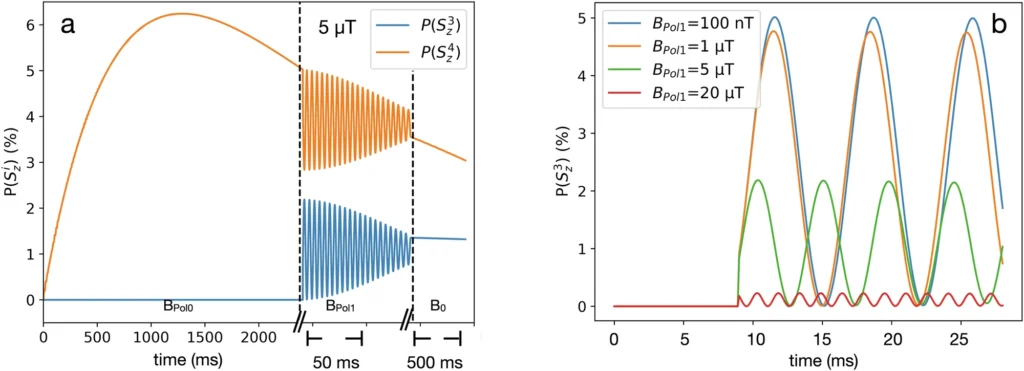
Prominent research and case studies:
Multiple investigations have demonstrated the effective polarization of lactic acid using PHIP, indicating the potential of this technology in a variety of applications. Research findings suggest that lactic acid is more susceptible to effective polarization due to its chemical nature. Comparative analyses have demonstrated that lactic acid can attain superior levels of polarization, rendering it a significant asset in both medicinal and industrial settings. The field has made significant advancements, including the creation of novel catalysts and reaction conditions that enhance the effectiveness of polarization transfer. Additionally, metabolic investigations and chemical analysis have successfully utilized polarized lactic acid.
Prospects for Future Research:
The subject of nuclear spin polarization is undergoing significant development, with continuous research focused on enhancing the efficiency and versatility of techniques such as PHIP. Current trends include the development of novel catalysts and strategies for parahydrogen production and purification, as well as advancements in experimental configurations and measurement methodologies. We anticipate future studies to explore novel applications of polarized lactic acid, including its potential in environmental monitoring and materials science. The potential for technical breakthroughs and novel discoveries in this subject is vast, offering substantial advantages across many scientific and industrial sectors.
In conclusion:
Nuclear Spin Polarization, The approach of exchanging parahydrogen-polarized protons to achieve nuclear spin polarization in lactic acid is a pioneering method with extensive ramifications. This technique improves the sensitivity of MRI and NMR imaging, resulting in more precise and detailed data for medical diagnostics, chemical analysis, and industrial processes. While challenges remain, we anticipate ongoing research and technological advancements to overcome these obstacles, leading to the creation of innovative applications and discoveries. The potential benefits of this Nuclear Spin Polarization are vast, allowing us to make significant progress in our comprehension and talents across a variety of domains.
Frequently Asked Questions:
1). What is nuclear spin polarization?
Nuclear spin polarization refers to the process of aligning the nuclear spins of atoms in a substance, which enhances its magnetic characteristics and enables its detection using MRI and NMR.
2). What’s the mechanism behind parahydrogen-induced polarization?
The PHIP method works by moving the spin polarization from parahydrogen to other molecules while they are reacting chemically. This makes their NMR signals much stronger.
3). What is lactic acid’s significance in medical research?
Lactic acid plays a vital role in the examination of metabolic processes and illnesses, as well as serving as a significant marker in several medical diagnostics, such as cancer and metabolic diseases.
4). What advantages does using parahydrogen provide for polarization?
Without the need for harsh conditions like low temperatures or strong magnetic fields, parahydrogen is an economically efficient substance that is simple to manufacture and can achieve significant polarization levels.
5). What future innovations might we anticipate in this domain?
Possible future developments may encompass enhanced catalysts, more effective parahydrogen generation techniques, and broader utilization in the medicinal, chemical, and industrial sectors.
For more chemistry blogs, visit chemistry Master