Table of Contents
Introduction to Palladium-Catalyzed Asymmetric Carbene Coupling:
An overview of the process known as palladium-catalyzed asymmetric carbene coupling.
Palladium-catalyzed asymmetric carbene coupling is a very important method in modern organic synthesis that has completely changed how complex molecular structures are built. This section explores the complexities of this catalytic process, providing insight into its importance and practical uses.
Definition and Importance:
Palladium-catalyzed asymmetric carbene coupling is a method for selectively making carbon-carbon bonds by adding carbenes to bonds that are not saturated. The reaction we talked about has become an important tool in the field of asymmetric synthesis because it makes it possible to make chiral compounds with a lot of control over their stereochemistry. The relevance of this rests in its capacity to optimize the production of physiologically active chemicals, natural goods, and innovative materials.
Significance in Organic Synthesis:
The ability to manipulate the stereochemistry of chemical reactions is critical in organic chemistry. The difficulty can be addressed by the utilization of palladium-catalyzed asymmetric carbene coupling, which offers scientists a diverse and efficient approach to synthesizing enantioenriched molecules. This technology has been used in palladium-catalyzed asymmetric carbene are extensively utilized in the domains of pharmaceutical chemistry, materials science, and natural product synthesis, leading to significant advancements and breakthroughs in these areas.
Background and Discovery:
An Overview of Carbene Coupling Reactions:
Palladium-catalyzed asymmetric Carbene coupling reactions are a type of chemical transformation in which carbenes, which are extremely reactive intermediates with a divalent carbon atom, are inserted into carbon-carbon or carbon-hydrogen bonds. This section explains the detailed mechanisms of carbene coupling reactions and their significance in chemical synthesis.
Mechanisms and Principles:
Palladium-catalyzed asymmetric Carbene coupling processes often involve the formation of carbenes from diazo compounds or other carbene precursors. Next, these carbenes go through the process of insertion into carbon-carbon bonds. This makes cyclopropane and other compounds like it. Steric hindrance, electronic effects, and the composition of the catalyst determine the stereochemistry of the reaction.
The role of carbenes in synthesis:
Carbenes are very flexible chemicals that are used in organic synthesis. They are very important in a lot of different reactions that create different bonds between carbon atoms and heteroatoms. Because carbenes can insert themselves into unsaturated bonds, carbene coupling reactions make it possible for new stereocenters to form. This property renders carbenes highly useful as fundamental components in the synthesis of intricate compounds.
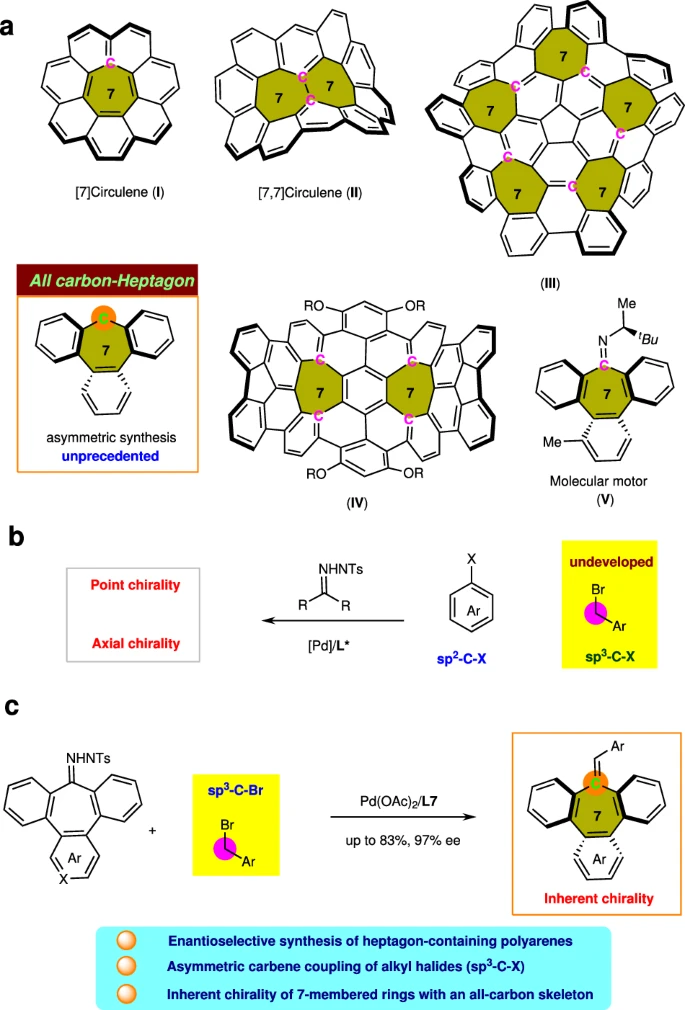
Pd-catalyzed carbene coupling between N-tosyl hydrazone with benzyl bromide:
The field of organic chemistry is characterized by chirality:
Organic chemistry relies heavily on chirality, the capacity of molecules to exist in two non-superimposed mirror-image configurations. This section examines the idea of chirality, its significance in chemical and biological systems, and the methods used to create enantioenriched substances.
Definition and Significance:
Chirality is caused by a lack of symmetry in a molecule’s spatial configuration of atoms. Enantiomers, which are the two mirror-image versions of a chiral molecule, can display significantly distinct characteristics, such as biological activity, toxicity, and pharmacokinetics. The capacity to manipulate the stereochemistry of chemical reactions is essential for synthesizing chiral molecules with specific characteristics.
Chiral Synthesis:
Enantioselective synthesis is a crucial approach for producing chiral molecules by selectively forming one enantiomer over the other. Using chiral catalysts, auxiliaries, or reagents, chemists can manipulate the stereochemistry of processes and achieve enantioenriched products with great selectivity. This method has completely transformed the process of creating medications, natural products, and fine chemicals.
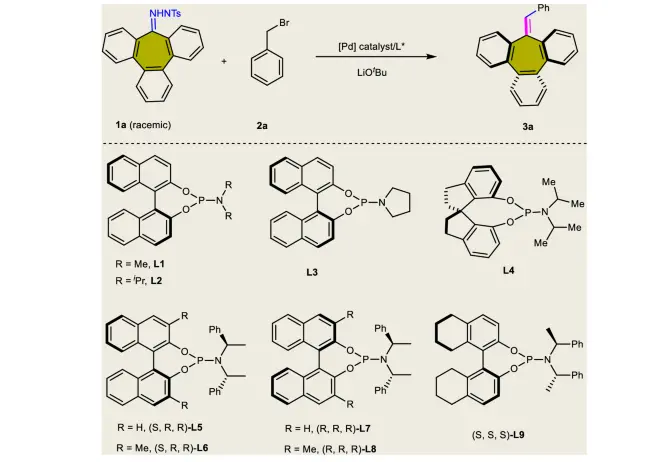
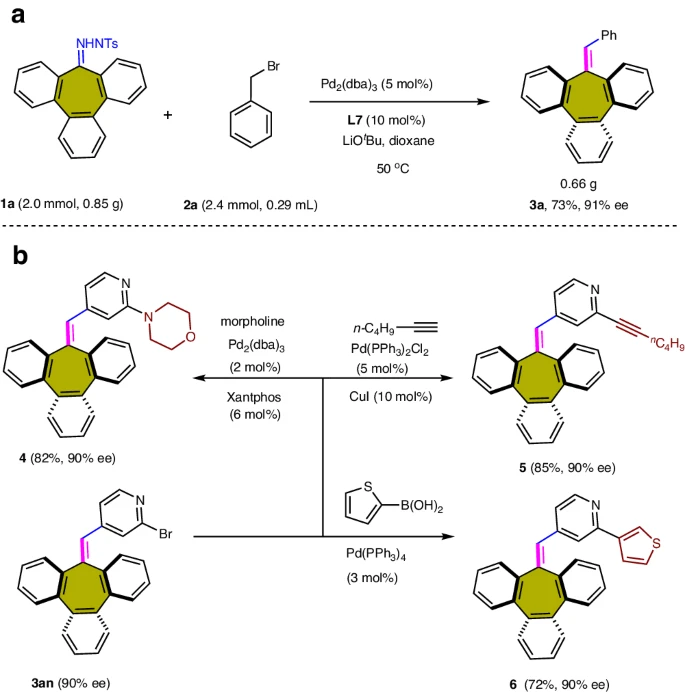
Synthetic applications of palladium-catalyzed carbene-based cross-coupling:
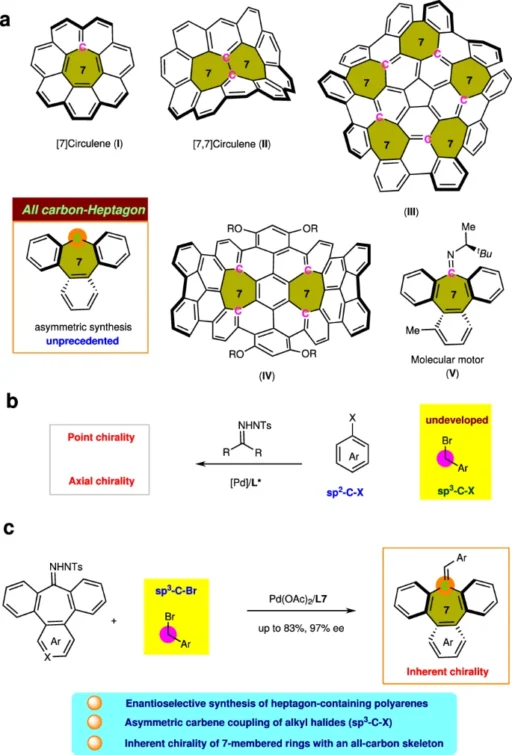
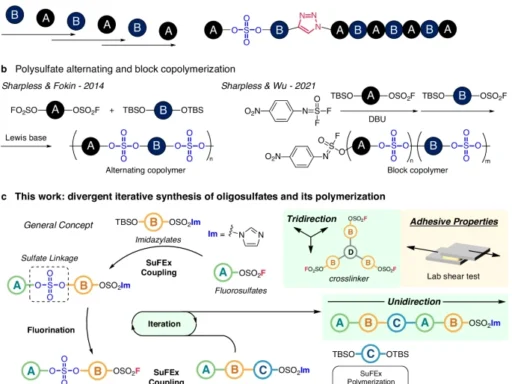
Structural insights into polyarenes containing heptagons:
Polyarenes are aromatic compounds that consist of several fused benzene rings. They display a wide range of structural patterns and electrical characteristics. Heptagon-containing polyarenes are a type of compound that has a seven-membered ring in its π-conjugated framework. These molecules have distinct structural and functional features that make them interesting.
Definition and Characteristics:
Heptagon-containing polyarenes, commonly referred to as heptanes or heptagons, are polycyclic aromatic hydrocarbons (PAHs) that have at least one ring consisting of seven carbon atoms. These molecules possess extended π-electron systems, which give them distinct optical, electrical, and magnetic properties. Heptagon-containing polyarenes have garnered considerable interest because of their potential uses in organic electronics, molecular sensing, and materials research.
Distinctive Characteristics and Utilizations:
When a heptagon is added to the π-conjugated structure of polyarenes, it changes their electronic structure and properties in big ways. Heptagon-containing polyarenes have better charge transport properties, less aromaticity, and different molecular packing arrangements compared to hexagonal polyarenes. The distinctive characteristics of these materials make them highly favorable options for use in organic field-effect transistors, light-emitting diodes, photovoltaic devices, and molecular switches.
Stereochemical stability studies of chiral tribenzocycloheptene compounds:
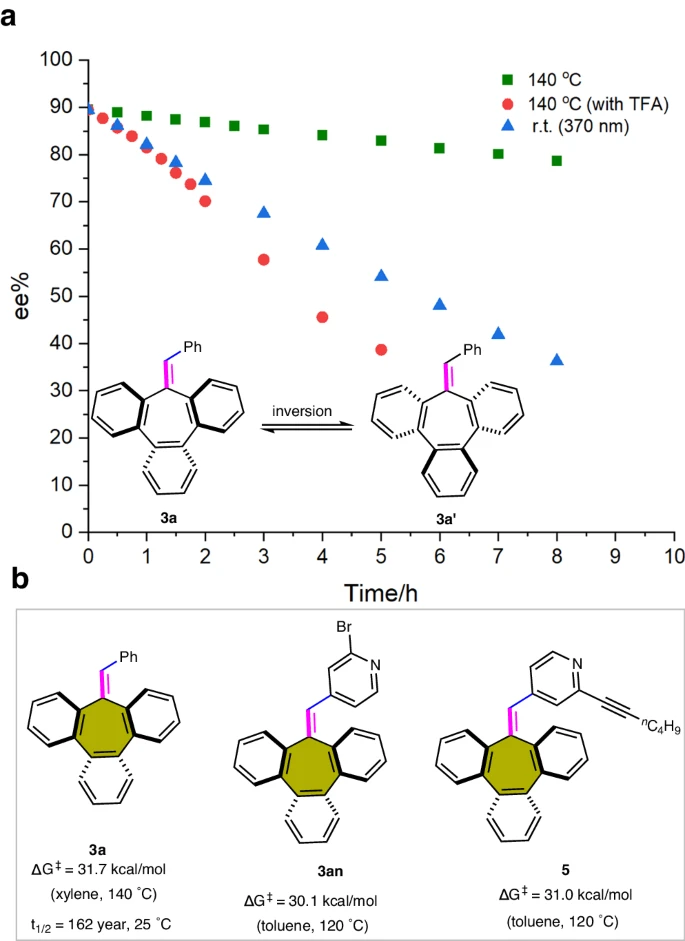
The Catalytic Power of Palladium:
When it comes to asymmetric carbene coupling processes, palladium catalysts are very good at speeding things up. This makes them very useful for making chiral compounds. This section offers a comprehensive introduction to palladium catalysts, including their reactivity and their wide range of uses in asymmetric synthesis.
Overview of Palladium Catalysts:
Chemical reactions benefit from the use of palladium catalysts. Palladium, a chemical element belonging to the platinum group of metals, composes them. Palladium catalysts are known for their high catalytic activity and selectivity, making them valuable.
Chemical synthesis extensively employs palladium catalysts due to their distinctive reactivity and adaptability. These catalysts can undertake oxidative addition, transmetalation, and reductive elimination processes. As a result, they can activate and modify a diverse set of organic substrates. For carbene coupling reactions, palladium catalysts work very well because they can help make carbon-carbon bonds under mild reaction conditions, showing great selectivity and efficiency.
Their Function in Asymmetric Synthesis:
Palladium catalysts are essential for regulating the stereochemistry of asymmetric carbene coupling processes. Chemists can selectively synthesize enantioenriched compounds by using chiral ligands or catalysts to favor the creation of one enantiomer over the other. This approach facilitates the synthesis of chiral compounds with a high degree of selectivity, rendering them a valuable asset in organic chemistry.
Recent Advances in Palladium-Catalyzed Carbene Coupling:
Recent years have seen significant advancements in the domain of palladium-catalyzed asymmetric carbene coupling. Progress in catalyst design, reaction techniques, and expanding the range of substances that can be used has resulted in the creation of effective and specific transformations. This provides synthetic chemists with new chances to produce intricate chiral compounds.
Significant advancements:
The most recent advancements in palladium-catalyzed carbene coupling have primarily focused on the development of novel chiral ligands and catalyst systems. These advancements have facilitated the production of a diverse array of enantioenriched products. These catalysts have very high levels of activity, selectivity, and compatibility with different functional groups. This makes it possible to make complex molecular structures with a lot of stereocenters very quickly.
Innovative Approaches and Practical Uses:
Aside from conventional palladium-catalyzed asymmetric carbene coupling processes, recent studies have investigated innovative approaches for the production of chiral compounds. These include cascade events, tandem processes, and multicomponent coupling reactions. They make it easy to quickly build complex molecular structures from simple starting materials. Natural products, medicines, and bioactive compounds have demonstrated the versatility and synthetic utility of palladium-catalyzed carbene coupling reactions.
Mechanistic studies:
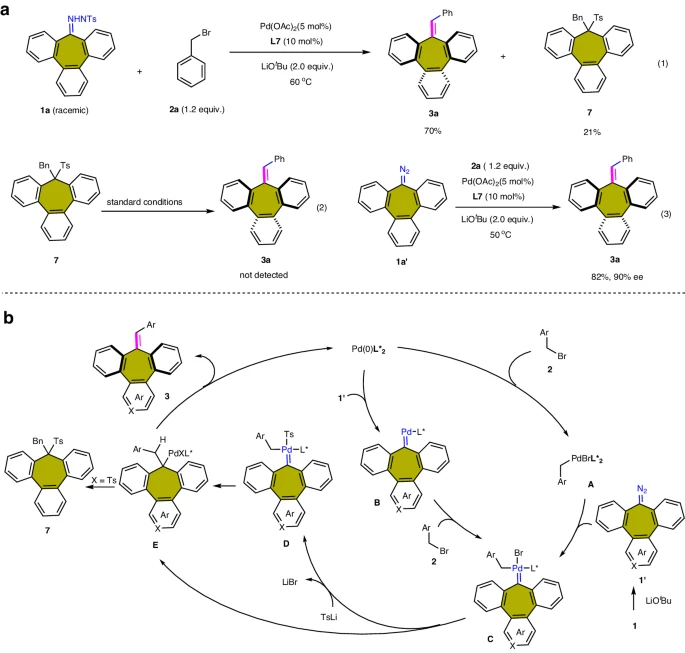
Obstacles and Prospects for the Future:
Despite the significant advancements in palladium-catalyzed asymmetric carbene coupling, this research area still faces some obstacles. These factors include substrate compatibility, the ability to adjust stereoselectivity, and the potential for scaling up reactions. To tackle these issues, it is necessary to foster interdisciplinary cooperation and devise innovative techniques to push the field toward new horizons.
Existing Challenges:
A major obstacle to palladium-catalyzed asymmetric carbene coupling is achieving a high degree of selectivity in intricate molecular systems. Substrate control, catalyst design, and reaction optimization are crucial determinants that impact the result of the reaction and the stereochemistry of the products. Also, using palladium-catalyzed asymmetric carbene coupling reactions in industry needs careful thought about how to make them work on a large scale and in real life, because the problems that come up are different from those that come up in the lab.
Possible avenues for research:
We expect subsequent investigations in the realm of palladium-catalyzed asymmetric carbene coupling to focus on many pivotal domains. These encompass the advancement of catalyst systems with enhanced activity, selectivity, and stability, along with the exploration of innovative reaction techniques and applications. Furthermore, endeavors to broaden the range of functional groups and substrates containing heteroatoms in carbene coupling reactions will augment the synthetic usefulness of this potent transformation.
Utilization of Polyarenes Containing Heptagons with Inherent Chirality:
Inherently chiral polyarenes containing heptagons are a captivating group of chemicals that have a wide range of uses in materials science, electronics, and biotechnology. Due to their distinctive structural motifs and electrical properties, they are very desirable for the creation of innovative materials and functional molecules with customized characteristics.
Substituent effect of benzyl bromide substrate 2:
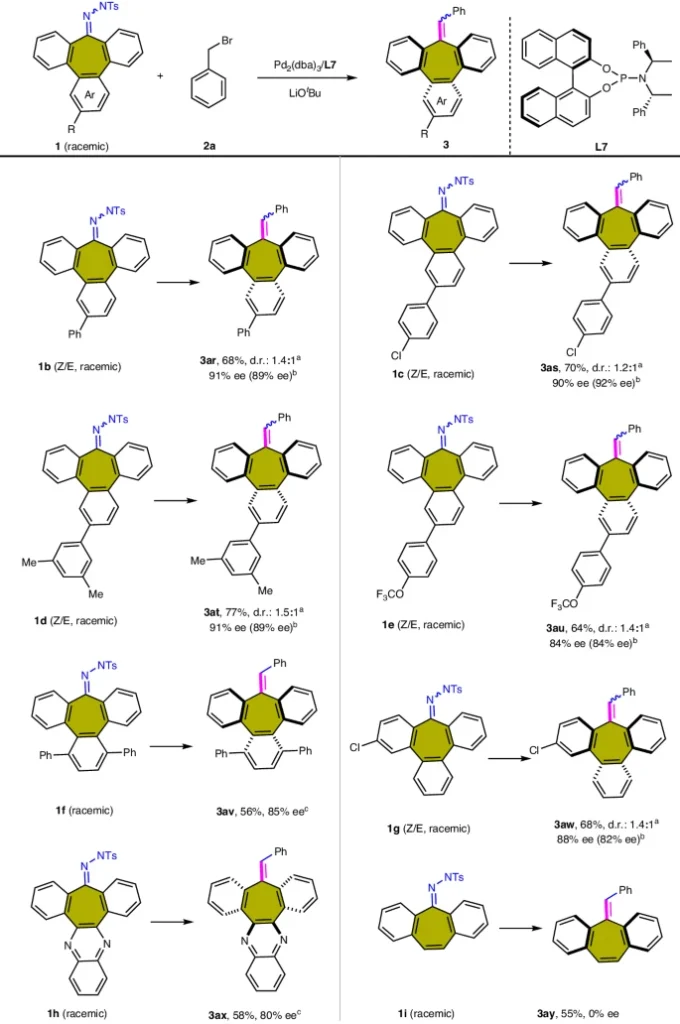
Application in Diverse Areas:
Heptagon-containing polyarenes have been utilized in several scientific fields, such as organic electronics, optoelectronic devices, and molecular sensing. Because of their ability to convey electrical charge, emit light, and interact with biomolecules, they are highly valuable instruments for the advancement of sophisticated materials and molecular electronics. Additionally, researchers have investigated polyarenes containing heptagons as chiral ligands and palladium-catalyzed asymmetric carbene synthesis. These compounds’ distinctive structural characteristics allow for the selective production of enantioenriched products.
Emerging opportunities and advancements:
The synthesis and characterization of polyarenes, including heptagons, have recently made significant progress, leading to a wider range of possible uses and usefulness. Thanks to improvements in synthesis methods, purification methods, and structural analysis tools, researchers have been able to get complex polyarenes with heptagons that are very pure and well-controlled. These advancements create opportunities to investigate new uses and characteristics of polyarenes that contain heptagons, as well as innovative methods for their production and modification.
In conclusion:
Overall, palladium-catalyzed asymmetric carbene coupling is a very good way to make complex chiral compounds, like polyarenes, which are made up of seven-membered rings that are naturally chiral. Through the utilization of the powerful catalytic abilities of palladium and the application of asymmetric synthesis principles, scientists can explore fresh possibilities in the fields of organic chemistry and materials science. This exploration can result in the creation of innovative materials, pharmaceuticals, and functional molecules that possess customized properties.
Frequently Asked Questions:
1). What sets palladium-catalyzed asymmetric carbene coupling apart from other synthetic methodologies?
Palladium-catalyzed asymmetric carbene coupling allows for precise manipulation of the stereochemistry involved in carbon-carbon bond formation. This process enables the creation of chiral compounds with remarkable levels of enantioselectivity, a feature rarely found in other synthetic processes.
2). What is the impact of palladium catalysts on carbene insertion stereochemistry?
Palladium-catalyzed asymmetric carbene enhances the reactivity of carbon-carbon π-bonds and interacts with the carbene intermediate, guiding its insertion into the intended site with the intended stereochemistry, thus permitting the production of enantioenriched products.
3). What are the main obstacles to attaining high levels of stereoselectivity in asymmetric carbene coupling reactions?
The challenges include regulating the orientation of the reacting partners, inhibiting competing reactions, and achieving efficient catalyst turnover while maintaining high degrees of enantioselectivity.
4). What are the potential applications of polyarenes incorporating heptagons in organic electronics?
Polyarenes that contain heptagons demonstrate advantageous electrical characteristics, including elevated charge carrier mobility and adjustable bandgaps. As a result, they are very prospective contenders for use in organic field-effect transistors, light-emitting diodes, and photovoltaic devices.
5). What impact do breakthroughs in asymmetric synthesis have on pharmaceutical development?
Palladium-catalyzed carbene coupling is an effective technique for asymmetric synthesis. It enables the creation of chiral intermediates and drug candidates with precise stereochemistry. This, in turn, improves the potency, selectivity, and pharmacokinetic aspects of pharmacological agents.
For more chemistry blogs, visit chemistry Master