Table of Contents
Preface of Photoluminescence:
Photoluminescence (PL) has transformed contemporary materials science, providing advancements in areas such as optoelectronics, bioimaging, and display technologies. Among many luminous materials, metal complexes, particularly aluminium-based compounds, have garnered significant interest owing to their adjustable features. Nonetheless, not all aluminium complexes demonstrate luminescence and the transition from non-luminescence to room-temperature phosphorescence (RTP) is a considerable hurdle. This article examines the regulation of photoluminescence in aluminium complexes through precise adjustment of metal substituents. Aluminium complexes were investigated in this study.
Comprehending Photoluminescence:
Photoluminescence is the light emission from a substance that occurs after photon absorption. We can classify this phenomenon into two principal types: fluorescence and phosphorescence. Fluorescence occurs almost instantly (in nanoseconds), while triplet excited states delay phosphorescence, leading to extended emission periods. Room-temperature phosphorescence (RTP), characterized by the ability of materials to phosphoresce at ambient temperatures, is particularly significant due to its potential for practical applications.
The significance of aluminium complexes in photoluminescent materials:
People value aluminium complexes for their stability, economic efficiency, and ease of synthesis. Due to their advantageous electrical properties, luminous materials, particularly organic electronics and displays, have acknowledged their significance. These complexes are utilized in photonic devices, including organic light-emitting diodes (OLEDs), where the efficiency of light emission is paramount.
The Challenge: Transitioning from Non-Luminescence to Phosphorescence
A notable problem with aluminium complexes is their frequent lack of luminescence under ambient conditions. Luminescence isn’t present when there isn’t enough spin-orbit coupling (SOC), there aren’t many energy gaps, or excited states quickly decay without giving off radiation. To get phosphorescence, especially room-temperature phosphorescence (RTP), in these materials, these problems have to be solved. Usually, this is done by changing the structure of the complex or adding metal substituents.
The Function of Metal Substituents in Modulating Luminescence:
Metal substituents play an important role in influencing the luminous properties of aluminium complexes. By changing the complex’s electronic structure, substitutes can change the energy levels, spin-orbit coupling, and dynamics of the excited state. Heavier atoms, like zinc or gallium, can augment spin-orbit coupling, facilitating intersystem crossover (ISC) and permitting phosphorescence. Alternative substituents may modify the ligand environment, enhancing stability and energy transfer efficiency. Photophysical properties of aluminium β-discriminate complexes.
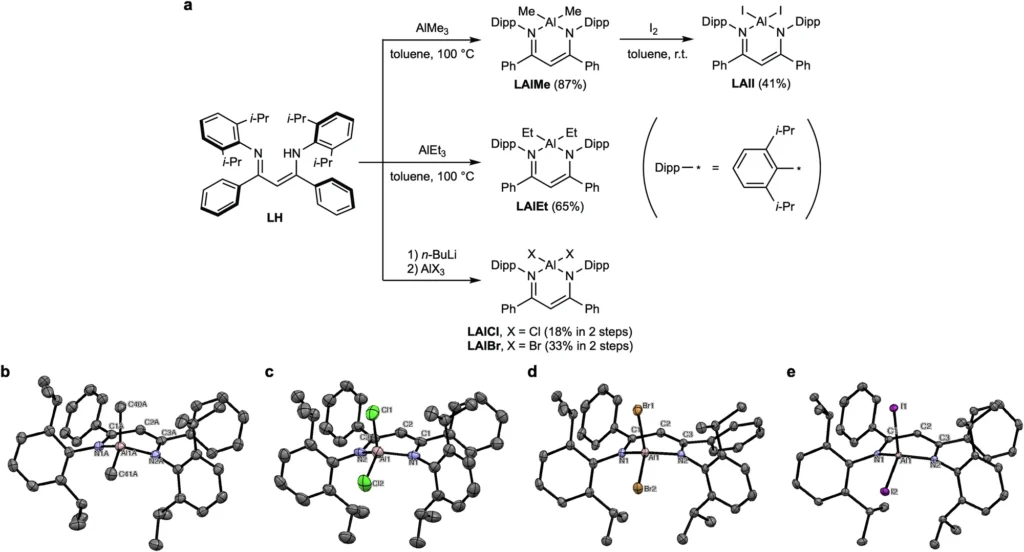
Methods for Attaining Room-Temperature Phosphorescence (RTP):
To achieve RTP in aluminium complexes, many techniques emphasize the incorporation of heavier metal substituents, which enhance SOC and ISC. The “heavy-atom effect” is important because elements with higher atomic numbers improve spin-orbit coupling, which makes triplet-state production more effective. Other methods include customizing ligands to keep excited states stable and changing the overall shape of the complex to stop non-radiative decay pathways.
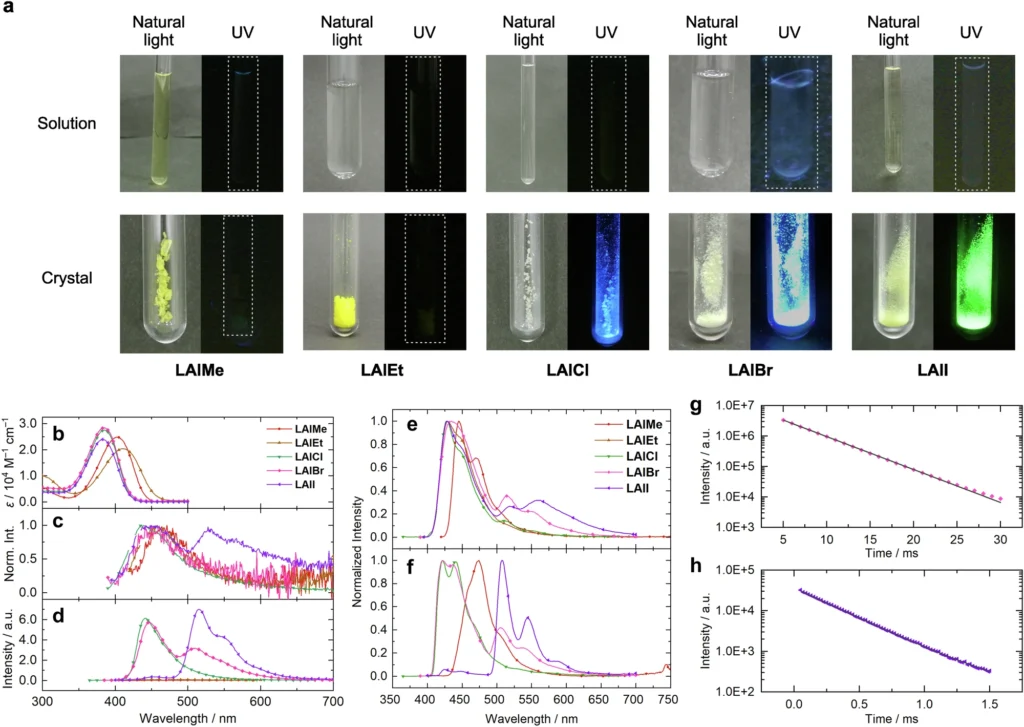
Recent Developments in Modulating Phosphorescence in Aluminium Complexes Case:
Study 1: Phosphorescence Attained Through Heavy Metal Substitution
One study showed that replacing lighter aluminium atoms with heavier metals such as platinum or iridium significantly enhanced RTP. These metals enabled intersystem crossing, converting hitherto non-luminescent complexes into effective phosphorescent materials.
Case Study 2: Ligand Modification and Its Impact on RTP
In a separate study, researchers altered the ligands around the aluminium centre, concentrating on augmenting the stiffness of the complex. This inhibited vibrational relaxation and non-radiative decay, thereby enhancing RTP efficiency. We have demonstrated that ligands with potent electron-withdrawing or donating groups affect triplet energy levels, thereby augmenting phosphorescence. Results of (TD-)DFT calculations.
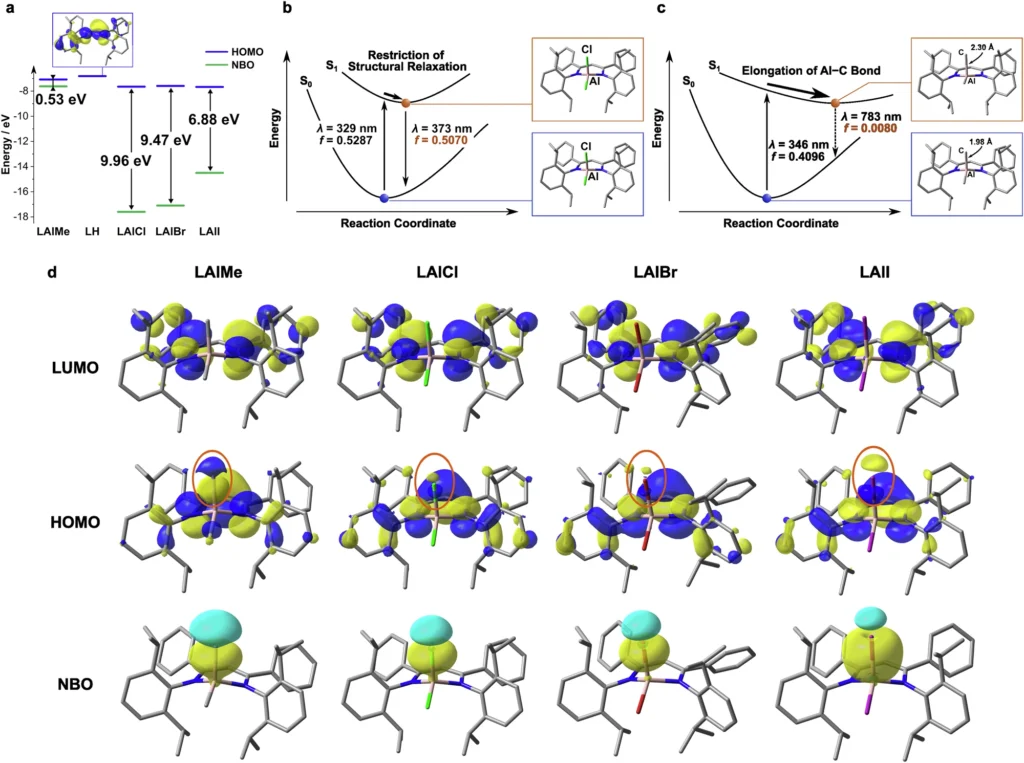
Utilisation of Aluminium Complexes with Controlled Phosphorescence:
Phosphorescent aluminium complexes, especially those exhibiting room-temperature phosphorescence (RTP), have several applications. RTP materials can markedly enhance the efficiency and longevity of OLEDs. In bioimaging, the prolonged emission from phosphorescent materials enhances contrast in imaging applications. Researchers are also exploring these complexes as sensors, taking advantage of their sensitivity to environmental fluctuations.
Obstacles in the Development of RTP Aluminium Complexes:
Notwithstanding their potential, the development of RTP aluminium complexes presents numerous hurdles. A primary challenge is the phosphorescent condition’s stability, particularly under environmental stress. Furthermore, attaining a balance between elevated phosphorescent efficiency and practical applicability (e.g., in displays or sensors) continues to pose challenges. Concerns exist about the environmental impact and safety of certain heavy metal substitutes used to enhance RTP.
Theoretical Insights: The Impact of Substituents on Photoluminescence
Theoretical models, including density functional theory (DFT), have yielded significant insights into the impact of metal substituents on photoluminescence. These models help predict the impact of various substituents on the electronic structure and excited states of aluminium complexes. Researchers can learn more about the relationship between structure and luminous properties by comparing theoretical predictions with actual data. This can help them make RTP materials that work better. Excited singlet and triplet states of the complexes.
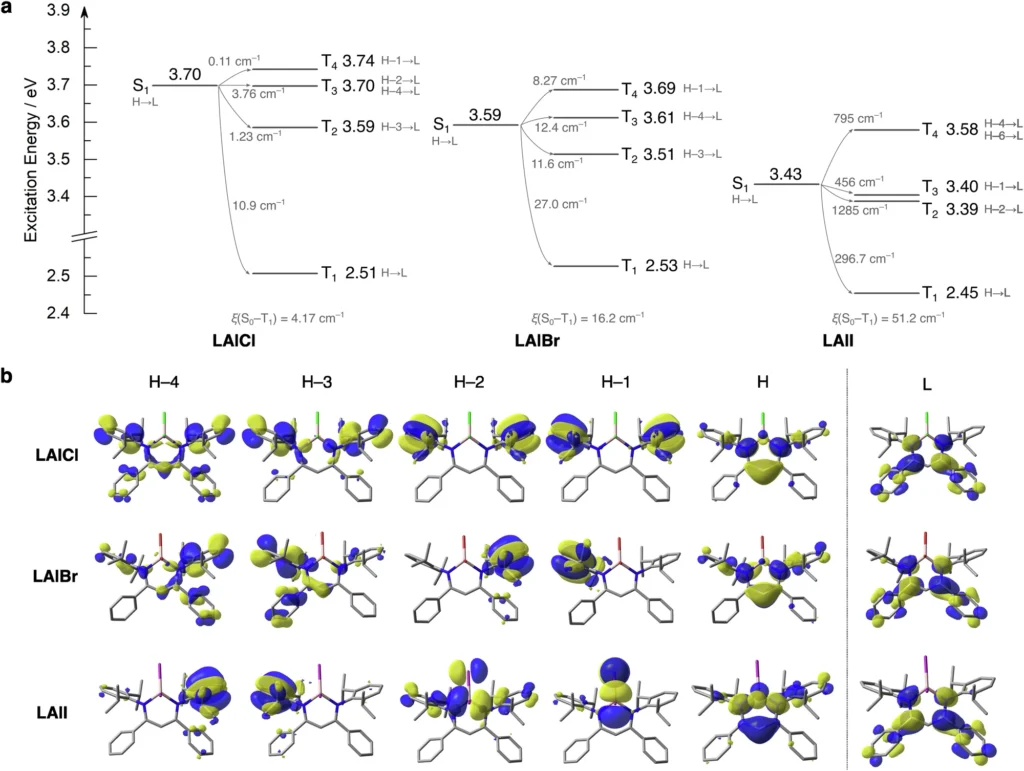
Prospective Avenues in Photoluminescent Aluminium Complex Investigation:
The future of photoluminescent aluminium complexes entails exploring next-generation materials that combine high efficiency with environmentally sustainable characteristics. Researchers are concentrating on environmentally friendly synthesis techniques, non-toxic metal alternatives, and enhanced room-temperature phosphorescence efficiency. Computational modelling will remain essential in forecasting the behaviour of novel complexes, while experimental endeavours will enhance the design of materials for wider applications.
Summary:
To sum up, changing the photoluminescence of aluminium complexes from not luminescent to phosphorescent at room temperature requires careful changes to the metal substituents. Researchers comprehending the impact of substituents on SOC, energy levels, and excited-state dynamics, researchers can create aluminium-based materials with exceptional phosphorescent characteristics. The future of this domain is expected to bring remarkable advancements, ranging from sophisticated OLEDs to novel applications in sensing and bioimaging.
Frequently Asked Questions:
1). What distinguishes fluorescence from phosphorescence?
Fluorescence occurs quickly, emitting light in nanoseconds, while phosphorescence often lasts for seconds due to the involvement of triplet states.
2). What is the significance of aluminium complexes in luminous materials?
Aluminium complexes are economically viable, stable, and exhibit advantageous electrical characteristics, rendering them suitable for applications in devices such as OLEDs.
3). What is the impact of metal substituents on the photoluminescence of aluminium complexes?
Adding metal substituents changes the electronic structure, which is important for phosphorescence because it increases spin-orbit coupling and intersystem crossover.
4). What are some of the practical applications of room-temperature phosphorescence?
OLEDs, bioimaging, and sensors use RTP materials to improve efficiency and contrast.
5). What challenges face the development of effective phosphorescent materials?
The principal issues are stability under climatic conditions, the equilibrium between efficiency and usefulness, and the assurance of safety for heavy metal substitutes.
For more chemistry blogs, visit chemistry Master